
Back Abakavir Afrikaans أباكافير Arabic اباکاویر AZB অ্যাবাকাভির Bengali/Bangla Abakavir Czech Abacafir Welsh Abacavir German Αβακαβίρη Greek Abacavir Spanish آباکاویر Persian
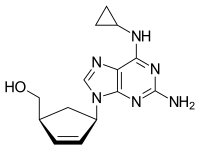 | |
 Chemical structure of abacavir | |
| Clinical data | |
|---|---|
| Pronunciation | /əˈbækəvɪər/ |
| Trade names | Ziagen |
| Other names | Abacavir sulfate (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699012 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 83% |
| Metabolism | Liver |
| Elimination half-life | 1.54 ± 0.63 h |
| Excretion | Kidney (1.2% abacavir, 30% 5'-carboxylic acid metabolite, 36% 5'-glucuronide metabolite, 15% unidentified minor metabolites). Fecal (16%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.341 |
| Chemical and physical data | |
| Formula | C14H18N6O |
| Molar mass | 286.339 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 165 °C (329 °F) |
| |
| |
| (verify) | |
Abacavir, sold under the brand name Ziagen among others, is a medication used to treat HIV/AIDS.[3][4][5][6] Similar to other nucleoside analog reverse-transcriptase inhibitors (NRTIs), abacavir is used together with other HIV medications, and is not recommended by itself.[7] It is taken by mouth as a tablet or solution and may be used in children over the age of three months.[5][8]
Abacavir is generally well tolerated.[8] Common side effects include vomiting, insomnia (trouble sleeping), fever, and feeling tired.[5] Other common side effects include loss of appetite, headache, nausea (feeling sick), diarrhea, rash, and lethargy (lack of energy).[4] More severe side effects include hypersensitivity, liver damage, and lactic acidosis.[5] Genetic testing can indicate whether a person is at higher risk of developing hypersensitivity.[5] Symptoms of hypersensitivity include rash, vomiting, and shortness of breath.[8] Abacavir is in the NRTI class of medications, which work by blocking reverse transcriptase, an enzyme needed for HIV virus replication.[9] Within the NRTI class, abacavir is a carbocyclic nucleoside.[5]
Abacavir was patented in 1988, and approved for use in the United States in 1998.[10][11] It is on the World Health Organization's List of Essential Medicines.[12] It is available as a generic medication.[5] Abacavir is used together with other HIV medications, such as abacavir/lamivudine/zidovudine, abacavir/dolutegravir/lamivudine, and abacavir/lamivudine.[8][9] The combination abacavir/lamivudine is an essential medicine.[12]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "TGA eBS - Product and Consumer Medicine Information Licence". Archived from the original on 24 March 2022. Retrieved 23 August 2022.
- ^ a b Cite error: The named reference
Ziagen FDA labelwas invoked but never defined (see the help page). - ^ a b c "Ziagen EPAR". European Medicines Agency. 17 September 2018. Archived from the original on 30 July 2022. Retrieved 22 August 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b c d e f g "Abacavir Sulfate". The American Society of Health-System Pharmacists. Archived from the original on 21 August 2017. Retrieved 31 July 2015.
- ^ "Drug Name Abbreviations Adult and Adolescent ARV Guidelines". AIDSinfo. Archived from the original on 9 November 2016. Retrieved 8 November 2016.
- ^ "What Not to Use Adult and Adolescent ARV Guidelines". AIDSinfo. Archived from the original on 9 November 2016. Retrieved 8 November 2016.
- ^ a b c d Yuen GJ, Weller S, Pakes GE (2008). "A review of the pharmacokinetics of abacavir". Clinical Pharmacokinetics. 47 (6): 351–371. doi:10.2165/00003088-200847060-00001. PMID 18479171. S2CID 31107341.
- ^ a b "Nucleoside reverse transcriptase inhibitors (NRTIs or 'nukes') - HIV/AIDS". www.hiv.va.gov. Archived from the original on 9 November 2016. Retrieved 8 November 2016.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 505. ISBN 9783527607495. Archived from the original on 8 September 2017.
- ^ Kane BM (2008). HIV/AIDS Treatment Drugs. Infobase Publishing. p. 56. ISBN 9781438102078. Archived from the original on 8 September 2017.
- ^ a b World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.