
Back انتقال إلكتروني Arabic Transició electrònica atòmica Catalan Elektronischer Übergang German Transición electrónica atómica Spanish Transition électronique French Transizione elettronica Italian 양자도약 Korean Transição eletrônica atômica Portuguese எதிர் மின்னணு தாவல் Tamil Атомний перехід електрона Ukrainian
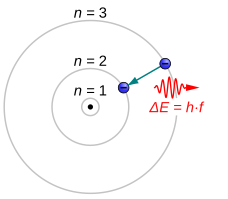
In atomic physics and chemistry, an atomic electron transition (also called an atomic transition, quantum jump, or quantum leap) is an electron changing from one energy level to another within an atom[1] or artificial atom.[2] The time scale of a quantum jump has not been measured experimentally. However, the Franck–Condon principle binds the upper limit of this parameter to the order of attoseconds.[3]
Electrons jumping to energy levels of smaller n emit electromagnetic radiation in the form of a photon. Electrons can also absorb passing photons, which drives a quantum jump to a level of higher n. The larger the energy separation between the electron's initial and final state, the shorter the photons' wavelength.[4]
- ^ Schombert, James. "Quantum physics" University of Oregon Department of Physics
- ^ Vijay, R; Slichter, D. H; Siddiqi, I (2011). "Observation of Quantum Jumps in a Superconducting Artificial Atom". Physical Review Letters. 106 (11): 110502. arXiv:1009.2969. Bibcode:2011PhRvL.106k0502V. doi:10.1103/PhysRevLett.106.110502. PMID 21469850. S2CID 35070320.
- ^ de la Peña, L.; Cetto, A. M.; Valdés-Hernández, A. (December 4, 2020). "How fast is a quantum jump?". Physics Letters A. 384 (34): 126880. arXiv:2009.02426. Bibcode:2020PhLA..38426880D. doi:10.1016/j.physleta.2020.126880. ISSN 0375-9601.
- ^ Cite error: The named reference
:0was invoked but never defined (see the help page).