
Back Benzilsäure-Umlagerung German Transposición del ácido bencílico Spanish نوآرایی بنزیلیک اسید Persian Bentsiilihappotoisiintuminen Finnish ベンジル酸転位 Japanese Бензиловая перегруппировка Russian பென்சிலிக் அமில மறுசீரமைப்பு Tamil Бензилове перегрупування Ukrainian 二苯乙醇酸重排反应 Chinese
The benzilic acid rearrangement is formally the 1,2-rearrangement of 1,2-diketones to form α-hydroxy–carboxylic acids using a base. This reaction receives its name from the reaction of benzil with potassium hydroxide to form benzilic acid. First performed by Justus von Liebig in 1838,[1] it is the first reported example of a rearrangement reaction.[2] It has become a classic reaction in organic synthesis and has been reviewed many times before.[3][4][5] It can be viewed as an intramolecular redox reaction, as one carbon center is oxidized while the other is reduced.
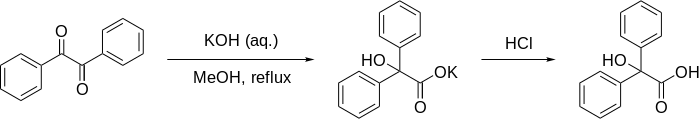
The reaction has been shown to work in aromatic, semi-aromatic, aliphatic, and heterocyclic substrates. The reaction works best when the ketone functional groups have no adjacent enolizable protons, as this allows aldol condensation to compete. The reaction is formally a ring contraction when used on cyclic diketones. It has been found that aryl groups more readily migrate than alkyl groups, and that aryl groups with electron-withdrawing groups migrate the fastest.
- ^ Liebig, J. (1838). "Ueber Laurent's Theorie der organischen Verbindungen". Annalen der Chemie. 25: 1–31. doi:10.1002/jlac.18380250102.
- ^ "Nerve Agent Precursors: Benzilic acid and Methyl Benzilate", Factsheets on Chemical and Biological Warfare Agents, Chemical precursors.
- ^ Selman, S.; Eastham, J. (1960). "Benzilic acid and related rearrangements". Q. Rev. Chem. Soc. 14 (3): 221–235. doi:10.1039/qr9601400221.
- ^ Bowden, K.; Fabien, W. M. F. (2001). "Reactions of carbonyl compounds in basic solutions. Part 36:The base-catalysed reactions of 1,2-dicarbonyl compounds". J. Phys. Org. Chem. 14 (11): 794–796. doi:10.1002/poc.433.
- ^ Gill, G. B. (1961). "Benzyl-benzilic acid rearrangements". Comp. Org. Synth. 3: 821–838.