 | |
| Clinical data | |
|---|---|
| Other names | GDC-0810, ARN-810, RG-6046, RO-7056118 |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
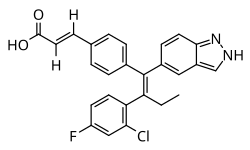
| Formula | C26H20ClFN2O2 |
| Molar mass | 446.91 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Brilanestrant (INN) (developmental code names GDC-0810, ARN-810, RG-6046, RO-7056118) is a nonsteroidal combined selective estrogen receptor modulator (SERM) and selective estrogen receptor degrader (SERD) that was discovered by Aragon Pharmaceuticals and was under development by Genentech for the treatment of locally advanced or metastatic estrogen receptor (ER)-positive breast cancer.[1][2][3][4][5]
Development of brilanestrant was discontinued by Roche in April 2017.[6] It reached phase II clinical trials for the treatment of breast cancer prior to the discontinuation of its development.[2][5]
- ^ "Proposed INN: List 115" (PDF). WHO Drug Information. 30 (2): 242–357. 2016.
- ^ a b "Drug Profile: GDC 0810". AdisInsight. 12 November 2016.
- ^ Lai A, Kahraman M, Govek S, Nagasawa J, Bonnefous C, Julien J, et al. (June 2015). "Identification of GDC-0810 (ARN-810), an Orally Bioavailable Selective Estrogen Receptor Degrader (SERD) that Demonstrates Robust Activity in Tamoxifen-Resistant Breast Cancer Xenografts". Journal of Medicinal Chemistry. 58 (12): 4888–904. doi:10.1021/acs.jmedchem.5b00054. PMID 25879485.
- ^ Joseph JD, Darimont B, Zhou W, Arrazate A, Young A, Ingalla E, et al. (July 2016). "The selective estrogen receptor downregulator GDC-0810 is efficacious in diverse models of ER+ breast cancer". eLife. 5: e15828. doi:10.7554/eLife.15828. PMC 4961458. PMID 27410477.
- ^ a b "Evaluating an ER Degrader for Breast Cancer". Cancer Discovery. 5 (7): OF15. July 2015. doi:10.1158/2159-8290.CD-NB2015-068. PMID 25956960.
- ^ John Carroll (27 April 2017). "Roche silently whisks away its $1.7B Seragon drug in a Q1 footnote". Endpoints News. Retrieved 27 April 2017.
