
Back أسيتات السيزيوم Arabic سزیوم استات AZB Caesiumacetat German سزیم استات Persian Cesiumasetaatti Finnish Cézium-acetát Hungarian Sesium asetat ID 酢酸セシウム Japanese സീസിയം അസറ്റേറ്റ് Malayalam Cesiumacetaat Dutch
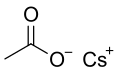 Structural formula
| |||
 Unit cell of anhydrous caesium acetate.
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Caesium acetate | |||
| Other names
Cesium acetate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.020.226 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H3CsO2 | |||
| Molar mass | 191.949 g/mol | ||
| Appearance | colourless, hygroscopic | ||
| Density | 2.423 g/cm3, solid | ||
| Melting point | 194 °C (381 °F; 467 K) | ||
| Boiling point | 945 °C (1,733 °F; 1,218 K) | ||
| 945.1 g/100 g (−2.5 °C) 1345.5 g/100 ml (88.5 °C) | |||
| Structure[2] | |||
| Primitve hexagonal | |||
| P6/m, No. 175 | |||
a = 1488.0 pm, c = 397.65 pm[2]
| |||
Lattice volume (V)
|
76.542 cm3·mol−1 | ||
Formula units (Z)
|
6 | ||
| Hazards | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions
|
Caesium formate | ||
Other cations
|
Lithium acetate Sodium acetate Potassium acetate Rubidium acetate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Caesium acetate or cesium acetate is an ionic caesium compound with the molecular formula CH3COOCs. It is a white solid that may be formed by the reaction of caesium hydroxide or caesium carbonate with acetic acid.[3]
- ^ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. B-91. ISBN 0-8493-0462-8..
- ^ a b Lossin, Adalbert; Meyer, Gerd (1993). "Kristallstruktur von Caesiumacetat, Cs(CH3COO)". Zeitschrift für Anorganische und Allgemeine Chemie. 619 (8): 1462–1464. doi:10.1002/zaac.19936190823.
- ^ Yode, Ryan (2015), "Cesium Acetate", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, pp. 1–11, doi:10.1002/047084289x.rn01845, ISBN 978-0-470-84289-8, retrieved 2020-07-21

