
Back کلرمادینون استات Persian Hlormadinon acetat Serbo-Croatian Hlormadinon acetat Serbian Chlormadinone acetate Vietnamese
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Belara, Lutéran, Prostal, others |
| Other names | CMA; RS-1280; ICI-39575; STG-155; NSC-92338; 17α-Acetoxy-6-chloro-6-dehydroprogesterone; 17α-Acetoxy-6-chloropregna-4,6-diene-3,20-dione |
| Routes of administration | By mouth[1] |
| Drug class | Progestogen; Progestin; Progestogen ester; Antigonadotropin; Steroidal antiandrogen |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100%[1][2][3] |
| Protein binding | 96.6–99.4% (to albumin and not to SHBG or CBG)[1][2] |
| Metabolism | Liver (reduction, hydroxylation, deacetylation, conjugation)[1][3] |
| Metabolites | • 3α-Hydroxy-CMA[4][1] • 3β-Hydroxy-CMA[4][1] • Others[1] |
| Elimination half-life | 25–89 hours[5][1][2][6] |
| Excretion | Urine: 33–45%[6][2] Feces: 24–41%[6][2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.563 |
| Chemical and physical data | |
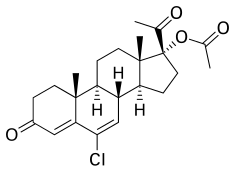
| Formula | C23H29ClO4 |
| Molar mass | 404.93 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women.[1][5][7][2][8][9][10] It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications.[11] It is taken by mouth.[1]
Side effects of the combination of an estrogen and CMA include menstrual irregularities, headaches, nausea, breast tenderness, vaginal discharge, and others.[2] At high dosages, CMA can cause sexual dysfunction, demasculinization, adrenal insufficiency, and changes in carbohydrate metabolism among other adverse effects.[12][13] The drug is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[1] It is also an antiandrogen, and hence is an antagonist of the androgen receptor, the biological target of androgens like testosterone and dihydrotestosterone.[1] Due to its progestogenic activity, CMA has antigonadotropic effects.[1][14][15] The medication has weak glucocorticoid activity and no other important hormonal activity.[1]
CMA was discovered in 1959 and was introduced for medical use in 1965.[16][17][18] It may be considered a "first-generation" progestin.[19] The medication was withdrawn in some countries in 1970 due to concerns about mammary toxicity observed in dogs, but this turned out not to apply to humans.[7][20][21][22][23] CMA is available widely throughout the world in birth control pills, but is notably not marketed in any predominantly English-speaking countries.[24][11] It is available alone in only a few countries, including France, Mexico, Japan, and South Korea.[24][11]
- ^ a b c d e f g h i j k l m n Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ a b c d e f g Raudrant D, Rabe T (2003). "Progestogens with antiandrogenic properties". Drugs. 63 (5): 463–92. doi:10.2165/00003495-200363050-00003. PMID 12600226. S2CID 28436828.
- ^ a b Lobo R, Crosignani PG, Paoletti R (31 October 2002). Women's Health and Menopause: New Strategies - Improved Quality of Life. Springer Science & Business Media. pp. 94–. ISBN 978-1-4020-7149-2.
- ^ a b Cite error: The named reference
pmid19590256was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Bouchard_2005was invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
pmid4206183was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
HughesHasan2013was invoked but never defined (see the help page). - ^ Mydlo JH, Godec CJ (11 July 2003). Prostate Cancer: Science and Clinical Practice. Academic Press. pp. 437–. ISBN 978-0-08-049789-1.
- ^ Kanimoto Y, Okada K (November 1991). "[Antiandrogen therapy of benign prostatic hyperplasia--review of the agents evaluation of the clinical results]". Hinyokika Kiyo (in Japanese). 37 (11): 1423–8. PMID 1722627.
- ^ Ishizuka O, Nishizawa O, Hirao Y, Ohshima S (November 2002). "Evidence-based meta-analysis of pharmacotherapy for benign prostatic hypertrophy". Int. J. Urol. 9 (11): 607–12. doi:10.1046/j.1442-2042.2002.00539.x. PMID 12534901. S2CID 8249363.
- ^ a b c Cite error: The named reference
Drugs.comwas invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid1693037was invoked but never defined (see the help page). - ^ Cite error: The named reference
pmid9712436was invoked but never defined (see the help page). - ^ Cite error: The named reference
BenniVemer1990was invoked but never defined (see the help page). - ^ Chassard D, Schatz B (2005). "[The antigonadrotropic activity of chlormadinone acetate in reproductive women]". Gynécologie, Obstétrique & Fertilité (in French). 33 (1–2): 29–34. doi:10.1016/j.gyobfe.2004.12.002. PMID 15752663.
- ^ Cite error: The named reference
Carp2015was invoked but never defined (see the help page). - ^ Patterson R (21 December 2012). Drugs in Litigation: Damage Awards Involving Prescription and Nonprescription Drugs. LexisNexis. pp. 184–. ISBN 978-0-327-18698-4.
- ^ Bud R, Finn BS, Trischler H (1999). Manifesting Medicine: Bodies and Machines. Taylor & Francis. pp. 113–. ISBN 978-90-5702-408-5.
- ^ Cite error: The named reference
Gordon_2007was invoked but never defined (see the help page). - ^ Cite error: The named reference
Lingeman2012was invoked but never defined (see the help page). - ^ Cite error: The named reference
StrefferBolt2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
Dallenbach-Hellweg2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
Gangolli2007was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Martindalewas invoked but never defined (see the help page).