
Back كلورتتراسكلين Arabic کلوترتراسایکلین AZB Chlortetracyklin Czech Chlortetracyclin German Clortetraciclina Spanish Klortetraziklina Basque کلرتتراسایکلین Persian Klooritetrasykliini Finnish Chlortétracycline French Բիոմիցին Armenian
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Aureomycin |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | By mouth, IV, topical |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 30% |
| Protein binding | 50 to 55% |
| Metabolism | Gastrointestinal tract, hepatic (75%) |
| Metabolites | Isochlortetracycline |
| Elimination half-life | 5.6 to 9 hours |
| Excretion | 60% renal and >10% biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | E702 (antibiotics) |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.310 |
| Chemical and physical data | |
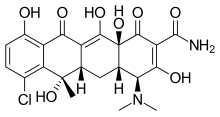
| Formula | C22H23ClN2O8 |
| Molar mass | 478.88 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | [α]D25−275°·cm3·dm−1·g−1 (methane) |
| Melting point | 168 to 169 °C (334 to 336 °F) |
| Solubility in water | 0.5–0.6 mg/mL (20 °C) |
| |
| |
| | |
Chlortetracycline (trade name Aureomycin, Lederle Laboratories) is a tetracycline antibiotic, the first tetracycline to be identified. It was discovered in 1945 at Lederle Laboratories under the supervision of scientist Yellapragada Subbarow, Benjamin Minge Duggar. They were helped by Louis T. Wright,[2] a surgeon who conducted this medications first human experiments. Duggar identified the antibiotic as the product of an actinomycete he cultured from a soil sample collected from Sanborn Field at the University of Missouri.[3] The organism was named Streptomyces aureofaciens and the isolated drug, Aureomycin, because of their golden color.[1]
It is on the World Health Organization's List of Essential Medicines.[4]
- ^ a b "chlortetracycline | C22H23ClN2O8 - PubChem". Pubchem.ncbi.nlm.nih.gov. Retrieved 2017-03-13.
- ^ Posner, Gerald. PHARMA : Greed, Lies, and the Poisoning of America. S.L., Avid Reader Pr, 2021, pp. 47–57.
- ^ Jukes TH (1985). "Some historical notes on chlortetracycline". Reviews of Infectious Diseases. 7 (5): 702–7. doi:10.1093/clinids/7.5.702. PMID 3903946.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.