
Back إعادة ترتيب كلايزن Arabic Claisenův přesmyk Czech Claisen-Umlagerung German Transposición de Claisen Spanish بازآرایی کلایزن Persian Claisen-toisiintuminen Finnish Réarrangement de Claisen French Կլայզենի վերախմբավորում Armenian Trasposizione di Claisen Italian クライゼン転位 Japanese
| Claisen rearrangement | |
|---|---|
| Named after | Rainer Ludwig Claisen |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| Organic Chemistry Portal | claisen-rearrangement |
| RSC ontology ID | RXNO:0000148 |
The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen.[1] The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation (Δ(ΔfH) = −327 kcal/mol (−1,370 kJ/mol).[2][3][4][5]
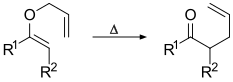
- ^ Cite error: The named reference
Claisenwas invoked but never defined (see the help page). - ^ Hiersemann, M.; Nubbemeyer, U. (2007) The Claisen Rearrangement. Wiley-VCH. ISBN 3-527-30825-3
- ^ Rhoads, S. J.; Raulins, N. R. (1975). "The Claisen and Cope Rearrangements". Org. React. 22: 1–252. doi:10.1002/0471264180.or022.01. ISBN 978-0471264187.
- ^ Ziegler, F. E. (1988). "The thermal, aliphatic Claisen rearrangement". Chem. Rev. 88 (8): 1423–1452. doi:10.1021/cr00090a001.
- ^ Wipf, P. (1991). "Claisen Rearrangements". Compr. Org. Synth. 5: 827–873. doi:10.1016/B978-0-08-052349-1.00140-2. ISBN 978-0-08-052349-1.