
Back كلوبازام Arabic کلوبازام AZB Klobazam Czech Clobazam German Clobazam Spanish کلوبازام Persian Klobatsaami Finnish Clobazam French קלובאזאם HE Klobazam Croatian
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Frisium, Urbanol, Onfi, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612008 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Benzodiazepine |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 87% (oral) |
| Protein binding | 80–90% |
| Metabolism | Liver |
| Metabolites |
|
| Onset of action | 0.5–4 hours |
| Elimination half-life |
|
| Excretion | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.810 |
| Chemical and physical data | |
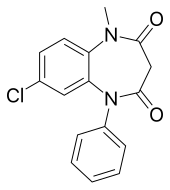
| Formula | C16H13ClN2O2 |
| Molar mass | 300.74 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Clobazam, sold under the brand names Frisium, Onfi and others, is a benzodiazepine class medication that was patented in 1968.[3] Clobazam was first synthesized in 1966 and first published in 1969. Clobazam was originally marketed as an anxioselective anxiolytic since 1970,[4][5] and an anticonvulsant since 1984.[6] The primary drug-development goal was to provide greater anxiolytic, anti-obsessive efficacy with fewer benzodiazepine-related side effects.[4]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Clobazam (T3D4564)". Toxin and Toxin Target Database (T3DB.
- ^ a b Humayun MJ, Samanta D, Carson RP (2020). "Clobazam". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 31082087.
- ^ Freche C (April 1975). "[Study of an anxiolytic, clobazam, in otorhinolaryngology in psychosomatic pharyngeal manifestations]". Semaine des Hopitaux. Therapeutique. 51 (4): 261–3. PMID 5777.
- ^ "Clobazam in treatment of refractory epilepsy: the Canadian experience. A retrospective study. Canadian Clobazam Cooperative Group". Epilepsia. 32 (3): 407–16. 1991. doi:10.1111/j.1528-1157.1991.tb04670.x. PMID 2044502. S2CID 24420211.