
Back تدفق سيتوبلازمي Arabic সাইটোপ্লাজমীয় প্রবাহ Bengali/Bangla Ciclosi Catalan Cytoplasmastrømninger Danish Zytoplasmatische Strömung German Ciclosis Spanish Cyclose French Cikloso IO Ciclosi Italian 原形質流動 Japanese
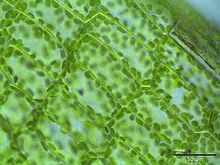
Cytoplasmic streaming, also called protoplasmic streaming and cyclosis, is the flow of the cytoplasm inside the cell, driven by forces from the cytoskeleton.[1] It is likely that its function is, at least in part, to speed up the transport of molecules and organelles around the cell. It is usually observed in large plant and animal cells, greater than approximately 0.1 mm[vague]. In smaller cells, the diffusion of molecules is more rapid, but diffusion slows as the size of the cell increases, so larger cells may need cytoplasmic streaming for efficient function.[1]
The green alga genus Chara possesses some very large cells, up to 10 cm in length,[2] and cytoplasmic streaming has been studied in these large cells.[3]
Cytoplasmic streaming is strongly dependent upon intracellular pH and temperature. It has been observed that the effect of temperature on cytoplasmic streaming created linear variance and dependence at different high temperatures in comparison to low temperatures.[4] This process is complicated, with temperature alterations in the system increasing its efficiency, with other factors such as the transport of ions across the membrane being simultaneously affected. This is due to cells homeostasis depending upon active transport which may be affected at some critical temperatures.
In plant cells, chloroplasts are transported within the cytoplasmic stream to optimize their exposure to light for photosynthesis.[5] This rate of motion is influenced by several factors including light intensity, temperature, and pH levels.[6] Cytoplasmic streaming is most efficient at a neutral pH and tends to decrease in efficiency under conditions of both low and high pH.[6]
Several methods exist to halt the flow of cytoplasm within cells. One approach involves the introduction of Lugol's iodine solution, which effectively immobilizes the cytoplasmic streaming.[citation needed] Alternatively, the compound Cytochalasin D, dissolved in dimethyl sulfoxide, can be employed to achieve a similar effect by disrupting the actin microfilaments responsible for facilitating cytoplasmic movement.[7]
- ^ a b Goldstein RE, van de Meent JW (August 2015). "A physical perspective on cytoplasmic streaming". Interface Focus. 5 (4): 20150030. doi:10.1098/rsfs.2015.0030. PMC 4590424. PMID 26464789.
- ^ Beilby MJ, Casanova MT (2013-11-19). The Physiology of Characean Cells. Springer Science & Business Media. ISBN 978-3-642-40288-3.
- ^ Woodhouse FG, Goldstein RE (August 2013). "Cytoplasmic streaming in plant cells emerges naturally by microfilament self-organization". Proceedings of the National Academy of Sciences of the United States of America. 110 (35): 14132–7. arXiv:1308.6422. Bibcode:2013PNAS..11014132W. doi:10.1073/pnas.1302736110. PMC 3761564. PMID 23940314.
- ^ Shimmen T, Yokota E (February 2004). "Cytoplasmic streaming in plants". Current Opinion in Cell Biology. 16 (1): 68–72. doi:10.1016/j.ceb.2003.11.009. PMID 15037307.
- ^ Bulychev AA, Dodonova SO (September 2011). "Effects of cyclosis on chloroplast-cytoplasm interactions revealed with localized lighting in Characean cells at rest and after electrical excitation". Biochimica et Biophysica Acta. 1807 (9): 1221–1230. doi:10.1016/j.bbabio.2011.06.009. PMID 21708122.
- ^ a b Goldstein RE, van de Meent JW (August 2015). "A physical perspective on cytoplasmic streaming". Interface Focus. 5 (4): 20150030. doi:10.1098/rsfs.2015.0030. PMC 4590424. PMID 26464789.
- ^ Foissner I, Wasteneys GO (March 1997). "A cytochalasin-sensitive actin filament meshwork is a prerequisite for local wound wall deposition in Nitella internodal cells". Protoplasma. 200 (1–2): 17–30. doi:10.1007/BF01280731. ISSN 0033-183X.