
Back ديكامبا Arabic Dicamba German Dikamba Estonian Dikamba Finnish Dicamba French Dicamba Galician Dicamba Dutch Дикамба Russian Dikamba Serbo-Croatian Dikamba Serbian
| |
| Names | |
|---|---|
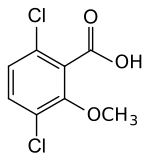
| Preferred IUPAC name
3,6-Dichloro-2-methoxybenzoic acid | |
| Other names
3,6-Dichloro-o-anisic acid
Dianat | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.016.033 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H6Cl2O3 | |
| Molar mass | 221.03 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 1.57 |
| Melting point | 114 to 116 °C (237 to 241 °F; 387 to 389 K) |
| "low"[2] | |
| Solubility in acetone | 810 g/L |
| Solubility in ethanol | 922 g/L |
| Hazards | |
| GHS labelling:[3] | |
 
| |
| Danger | |
| H302, H318, H412 | |
| P273, P280, P305+P351+P338 | |
| Flash point | 199 °C (390 °F; 472 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dicamba (3,6-dichloro-2-methoxybenzoic acid) is a selective systemic herbicide first registered in 1967.[4] Brand names for formulations of this herbicide include Dianat, Banvel, Diablo, Oracle and Vanquish. This chemical compound is a chlorinated derivative of o-anisic acid.[5] It has been described as a "widely used, low-cost, environmentally friendly herbicide that does not persist in soils and shows little or no toxicity to wildlife and humans."[6]
Despite its success in improving crop yields, dicamba has attracted controversy. According to the United States Environmental Protection Agency (EPA), dicamba's primary ecological risk is for non-target terrestrial plants from exposure through spray drift, whereby dicamba inadvertently migrates to non-targeted neighboring areas, damaging those plants.[7][8]
In 2016, dicamba was approved for use in the United States over GMO dicamba-resistant crops created by Monsanto. Dicamba came under significant scrutiny due to its tendency to spread from treated fields into neighboring fields, causing damage.[9] The controversy led to litigation, state bans and additional restrictions over dicamba use.
- ^ Merck Index, 11th Edition, 3026.
- ^ Cite error: The named reference
Ullmannwas invoked but never defined (see the help page). - ^ Record of Dicamba in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2023-12-12.
- ^ "Reregistration Eligibility Decision for Dicamba and Associated Salts - US EPA" (PDF). 8 June 2006.
- ^ "Dicamba (Banvel) Herbicide Profile 10/83, Pesticide Management Education Program". Cornell University.
- ^ Cite error: The named reference
Weekswas invoked but never defined (see the help page). - ^ "Dicamba". United States Environmental Protection Agency. 16 February 2023.
- ^ "Dicamba". United States Environmental Protection Agency. 16 February 2023. Archived from the original on 23 December 2023.
- ^ Revealed: Monsanto predicted crop system would damage US farms The Guardian, 2020