
Back درونيدارون Arabic Dronedaron German Dronedarona Spanish دروندارون Persian Dronédarone French Dronedarone Italian 드로네다론 Korean ଡ୍ରୋନଡାରୋନ OR Dronedaron Serbo-Croatian Dronedaron Serbian
 | |
| Clinical data | |
|---|---|
| Trade names | Multaq |
| Other names | SR33589 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609034 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 15% (with a high-fat meal)[2] |
| Protein binding | >98% |
| Metabolism | Liver (mainly by CYP3A) |
| Elimination half-life | 13–19 hours |
| Excretion | Feces (84%), urine (~6%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.109.411 |
| Chemical and physical data | |
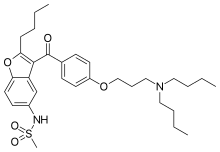
| Formula | C31H44N2O5S |
| Molar mass | 556.76 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dronedarone, sold under the brand name Multaq, is a class III antiarrhythmic medication developed by Sanofi-Aventis.[citation needed] It was approved by the US Food and Drug Administration (FDA) in July 2009.[citation needed] Besides being indicated in arrhythmias, it was recommended as an alternative to amiodarone for the treatment of atrial fibrillation and atrial flutter in people whose hearts have either returned to normal rhythm or who undergo drug therapy or electric shock treatment i.e. direct current cardioversion (DCCV) to maintain normal rhythm.[medical citation needed] It is a class III antiarrhythmic drug.[4] The FDA label includes a claim for reducing hospitalization, but not for reducing mortality, as a reduction in mortality was not demonstrated in the clinical development program.[5] A trial of the drug in heart failure was stopped as an interim analysis showed a possible increase in heart failure deaths, in people with moderate to severe congestive heart failure.[6]
The FDA label for dronedarone includes a boxed warning, stating that dronedarone is contraindicated in patients with NYHA Class IV heart failure, NYHA Class II and III heart failure with a recent decompensation requiring hospitalization or referral to a specialized heart failure clinic, or with permanent atrial fibrillation."[2] Dronedarone is also associated with rare cases of severe liver damage, including liver failure.[7]
It is approved as a generic medication.[8]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved October 22, 2023.
- ^ a b c "Multaq- dronedarone tablet, film coated". DailyMed. October 15, 2020. Retrieved November 18, 2020.
- ^ "Multaq EPAR". European Medicines Agency (EMA). November 26, 2009. Retrieved September 6, 2024.
- ^ "FDA Approves Multaq to Treat Heart Rhythm Disorder" (Press release). U.S. Food and Drug Administration (FDA). July 2, 2009. Archived from the original on July 4, 2009. Retrieved July 2, 2009.
- ^ Zimetbaum PJ (April 2009). "Dronedarone for atrial fibrillation--an odyssey". The New England Journal of Medicine. 360 (18): 1811–1813. doi:10.1056/NEJMp0902248. PMID 19403901.
- ^ Køber L, Torp-Pedersen C, McMurray JJ, Gøtzsche O, Lévy S, Crijns H, et al. (June 2008). "Increased mortality after dronedarone therapy for severe heart failure". The New England Journal of Medicine. 358 (25): 2678–2687. doi:10.1056/NEJMoa0800456. PMID 18565860.
- ^ "FDA Drug Safety Communication: Severe liver injury associated with the use of dronedarone (marketed as Multaq). Safety Announcement". U.S. Food and Drug Administration (FDA). January 14, 2011.
- ^ "First-Time Generic Drug Approvals 2024". U.S. Food and Drug Administration (FDA). March 8, 2024. Retrieved March 9, 2024.