
Back دولوكسيتين Arabic دولوکستین AZB Duloxetin Danish Duloxetin German Ντουλοξετίνη Greek Duloxetina Spanish دولوکستین Persian Duloksetiini Finnish Duloxétine French דולוקסטין HE
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cymbalta, Ariclaim, Yentreve, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604030 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Serotonin–norepinephrine reuptake inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~ 50% (32% to 80%) |
| Protein binding | ~ 95% |
| Metabolism | Liver, two P450 isozymes, CYP2D6 and CYP1A2 |
| Elimination half-life | 12 hours |
| Excretion | 70% in urine, 20% in feces |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.116.825 |
| Chemical and physical data | |
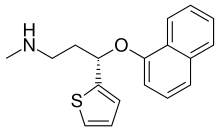
| Formula | C18H19NOS |
| Molar mass | 297.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Duloxetine, sold under the brand name Cymbalta among others,[1] is a medication used to treat major depressive disorder, generalized anxiety disorder, obsessive-compulsive disorder, fibromyalgia, neuropathic pain and central sensitization.[7][8] It is taken by mouth.[7]
Duloxetine is a serotonin–norepinephrine reuptake inhibitor (SNRI).[9] Similarly to SSRIs and other SNRIs, the precise mechanism for its antidepressant and anxiolytic effects is not known.[7]
Common side effects include dry mouth, nausea, feeling tired, dizziness, agitation, sexual problems, and increased sweating.[7] Severe side effects include an increased risk of suicide, serotonin syndrome, mania, and liver problems.[7] Antidepressant withdrawal syndrome may occur if stopped.[7] There are concerns that use during the later part of pregnancy can harm the developing fetus.[7]
Duloxetine was approved for medical use in the United States and in the European Union in 2004.[5][6][7] It is available as a generic medication.[9] In 2022, it was the 31st most commonly prescribed medication in the United States, with more than 18 million prescriptions.[10][11]
- ^ a b "Duloxetine". Drugs.com. Archived from the original on 29 September 2018. Retrieved 24 December 2018.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). 31 March 2023. Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ Cite error: The named reference
DailyMed-2020was invoked but never defined (see the help page). - ^ a b "Cymbalta EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 20 October 2020. Retrieved 30 September 2020.
- ^ a b Cite error: The named reference
EMA-2018-EPARwas invoked but never defined (see the help page). - ^ a b c d e f g h "Duloxetine". Monograph. The American Society of Health-System Pharmacists. Archived from the original on 26 November 2018. Retrieved 24 December 2018.
- ^ "Medications for OCD". International OCD Foundation. Archived from the original on 27 January 2024. Retrieved 25 February 2024.
- ^ a b British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 364–365. ISBN 9780857113382.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Duloxetine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Archived from the original on 12 April 2020. Retrieved 30 August 2024.