
Back إنفلوران Arabic انفلوران AZB Enfluran Czech Enfflwran Welsh Enfluran German Enflurano Spanish انفلوران Persian Enfluraani Finnish Enflurane French Enflurano Italian
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 97% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.034.126 |
| Chemical and physical data | |
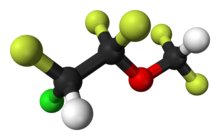
| Formula | C3H2ClF5O |
| Molar mass | 184.49 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Enflurane (2-chloro-1,1,2-trifluoroethyl difluoromethyl ether) is a halogenated ether. Developed by Ross Terrell in 1963, it was first used clinically in 1966. It was increasingly used for inhalational anesthesia during the 1970s and 1980s[2] but is no longer in common use.[3]
Enflurane is a structural isomer of isoflurane. It vaporizes readily, but is a liquid at room temperature.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Niedermeyer E, Lopes da Silva FH (2005). Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Lippincott Williams & Wilkins. p. 1156. ISBN 978-0-7817-5126-1.
- ^ Hemmings Jr HC, Egan TD (2013). Pharmacology and Physiology for Anesthesia. doi:10.1016/C2009-0-41712-4. ISBN 9781437716795.