
Back হফ্ম্যান ক্ষুদ্রাংশকরণ Bengali/Bangla Transposició de Hofmann Catalan Hofmannův přesmyk Czech Hofmann-Umlagerung German Transposición de Hofmann Spanish بازآرایی هافمن Persian Hofmann-toisiintuminen Finnish Réarrangement de Hofmann French Hofmann-lebontás Hungarian Հոֆմանի ռեակցիաներ Armenian
| Hofmann rearrangement | |
|---|---|
| Named after | August Wilhelm von Hofmann |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000410 |
The Hofmann rearrangement (Hofmann degradation) is the organic reaction of a primary amide to a primary amine with one less carbon atom.[1][2][3] The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines.
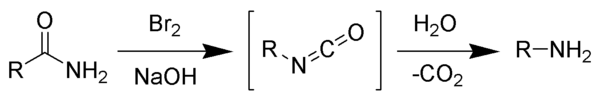
The reaction is named after its discoverer, August Wilhelm von Hofmann, and should not be confused with the Hofmann elimination, another name reaction for which he is eponymous.
- ^ Hofmann, A. W. (1881). "Ueber die Einwirkung des Broms in alkalischer Lösung auf Amide" [On the action of bromine in alkaline solution on amides]. Berichte der Deutschen Chemischen Gesellschaft. 14 (2): 2725–2736. doi:10.1002/cber.188101402242.
- ^ Everett, Wallis; Lane, John (1946). The Hofmann Reaction. Vol. 3. pp. 267–306. doi:10.1002/0471264180.or003.07. ISBN 9780471005285.
{{cite book}}:|journal=ignored (help) - ^ Shioiri, Takayuki (1991). "Degradation Reactions". Comprehensive Organic Synthesis. Vol. 6. pp. 795–828. doi:10.1016/B978-0-08-052349-1.00172-4. ISBN 9780080359298.