
Back أسيتات الحديد الثلاثي Arabic استات دمیر (III) AZB Octan železitý Czech Fera (III) acetato Esperanto استات آهن (III) Persian Rauta(III)asetaatti Finnish IJzer(III)acetaat Dutch Acetato de ferro(III) Portuguese Gvožđe(III) acetat Serbo-Croatian Gvožđe(III) acetat Serbian
| |
| |
| Names | |
|---|---|
| IUPAC name
iron(III) acetate
| |
| Other names
basic iron(III) acetate , iron(III) oxyacetate, iron(III) Acetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H27Fe3O18 | |
| Molar mass | 650.9 g/mol |
| Appearance | brownish-red powder |
| Insoluble | |
| Solubility | soluble in ethanol[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
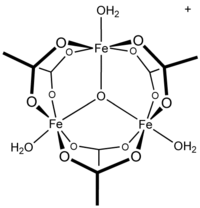
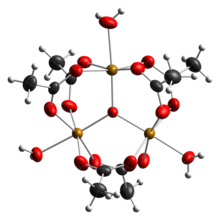
Ferric acetate is the acetate salt of the coordination complex [Fe3O(OAc)6(H2O)3]+ (OAc− is CH3CO2−). Commonly the salt is known as "basic iron acetate".[3] The formation of the red-brown complex was once used as a test for ferric ions.[4]
- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. pp. 4–63. ISBN 0-8493-0487-3.
- ^ "Iron(III) Acetate". EndMemo. Retrieved 18 April 2015.
- ^ J., Burgess; M. V., Twigg (2005). R. Bruce, King; J., Wiley (eds.). Encyclopedia of inorganic chemistry (2nd ed.). New York: Wiley. ISBN 978-0-470-86078-6.
- ^ H., Brearley; F., Ibbotson (1902). The Analysis of Steel-Works Materials. London ; New York: Longmans, Green. Archived from the original on 18 April 2015.