
Back Tolmanův úhel Czech Ligandenkegelwinkel German Ángulo de Tolman Spanish Լիգանդի կոնային անկյուն Armenian Angolo conico di Tolman Italian 配位子円錐角 Japanese Kegelhoek Dutch Конический угол лиганда Russian
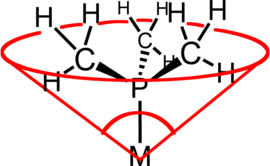
In coordination chemistry, the ligand cone angle (θ) is a measure of the steric bulk of a ligand in a transition metal coordination complex. It is defined as the solid angle formed with the metal at the vertex of a cone and the outermost edge of the van der Waals spheres of the ligand atoms at the perimeter of the base of the cone. Tertiary phosphine ligands are commonly classified using this parameter, but the method can be applied to any ligand. The term cone angle was first introduced by Chadwick A. Tolman, a research chemist at DuPont. Tolman originally developed the method for phosphine ligands in nickel complexes, determining them from measurements of accurate physical models.[1][2][3]
- ^ Tolman, Chadwick A. (1970-05-01). "Phosphorus ligand exchange equilibriums on zerovalent nickel. Dominant role for steric effects". J. Am. Chem. Soc. 92 (10): 2956–2965. doi:10.1021/ja00713a007.
- ^ Tolman, C. A.; Seidel, W. C.; Gosser, L. W. (1974-01-01). "Formation of three-coordinate nickel(0) complexes by phosphorus ligand dissociation from NiL4". J. Am. Chem. Soc. 96 (1): 53–60. doi:10.1021/ja00808a009.
- ^ Tolman, C. A. (1977). "Steric Effects of Phosphorus Ligands in Organometallic Chemistry and Homogeneous Catalysis". Chem. Rev. 77 (3): 313–48. doi:10.1021/cr60307a002.