Back يوديد المغنيسيوم Arabic منیزیوم یودیود AZB ম্যাগনেসিয়াম আয়োডাইড Bengali/Bangla Iodur de magnesi Catalan Jodid hořečnatý Czech Magnesiumiodid German Magnezia jodido Esperanto منیزیم یدید Persian Magnesiumjodidi Finnish Iodure de magnésium French
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Magnesium iodide
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.030.738 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| |||
| Molar mass |
| ||
| Appearance | white crystalline solid | ||
| Odor | odorless | ||
| Density |
| ||
| Melting point | 637 °C (1,179 °F; 910 K) (anhydrous, decomposes) 41 °C (octahydrate, decomposes) | ||
| |||
| Solubility | soluble in ether, alcohol and ammonia | ||
| −111.0·10−6 cm3/mol | |||
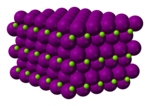
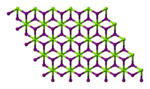
| Structure | |||
| |||
| Thermochemistry | |||
Heat capacity (C)
|
74 J/(mol·K) | ||
Std molar
entropy (S⦵298) |
134 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−364 kJ/mol | ||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H315, H319 | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other anions
|
|||
Other cations
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Magnesium iodide is an inorganic compound with the chemical formula MgI2. It forms various hydrates MgI2·xH2O. Magnesium iodide is a salt of magnesium and hydrogen iodide. These salts are typical ionic halides, being highly soluble in water.
- ^ Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, p. 240, ISBN 0-8493-8671-3, retrieved 2007-12-09
- ^ Magnesium Iodide MSDS at AlfaAesar[permanent dead link]