
Back Magnesiumsulfied Afrikaans كبريتيد المغنيسيوم Arabic منیزیوم سولفید AZB Сульфід магнію Byelorussian ম্যাগনেসিয়াম সালফাইড Bengali/Bangla Sulfur de magnesi Catalan Sulfid hořečnatý Czech Magnesiumsulfid German Sulfuro de magnesio Spanish منیزیم سولفید Persian
| |
| Names | |
|---|---|
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.597 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| MgS | |
| Molar mass | 56.38 g/mol |
| Appearance | white to reddish brown powder |
| Density | 2.84 g/cm3 |
| Melting point | 2,000 °C (3,630 °F; 2,270 K) approx. |
| decomposes | |
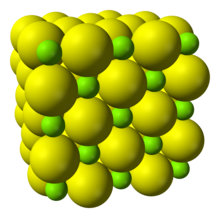
| Structure | |
| Halite (cubic), cF8 | |
| Fm3m, No. 225 | |
| cubic | |
| Thermochemistry | |
Heat capacity (C)
|
45.6 J/mol K |
Std molar
entropy (S⦵298) |
50.3 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
-347 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Source of H2S |
| Related compounds | |
Other anions
|
Magnesium oxide |
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Magnesium sulfide is an inorganic compound with the formula MgS. It is a white crystalline material but often is encountered in an impure form that is brown and non-crystalline powder. It is generated industrially in the production of metallic iron.