
Back كلوريد المنغنيز الثنائي Arabic کولورید منقنز (II) AZB Chlorid manganatý Czech Mangan(II)-chlorid German Mangana (II) klorido Esperanto Cloruro de manganeso(II) Spanish کلرید منگنز (II) Persian Mangaani(II)kloridi Finnish Chlorure de manganèse(II) French मैंगनिज(II) क्लोराइड Hindi
 ball-and-stick model of crystal packing
| |
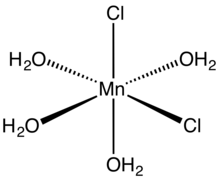 molecular structure
| |
 Tetrahydrate
| |
| Names | |
|---|---|
| IUPAC names
Manganese(II) chloride
Manganese dichloride | |
| Other names
Manganous chloride
hyperchloride of manganese | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.972 |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| MnCl2 | |
| Molar mass | 125.844 g/mol (anhydrous) 161.874 g/mol (dihydrate) 197.91 g/mol (tetrahydrate) |
| Appearance | pink solid (tetrahydrate) |
| Density | 2.977 g/cm3 (anhydrous) 2.27 g/cm3 (dihydrate) 2.01 g/cm3 (tetrahydrate) |
| Melting point | 654 °C (1,209 °F; 927 K) (anhydrous) dihydrate dehydrates at 135 °C tetrahydrate dehydrates at 58 °C |
| Boiling point | 1,225 °C (2,237 °F; 1,498 K) |
| 63.4 g/100 ml (0 °C) 73.9 g/100 ml (20 °C) 88.5 g/100 ml (40 °C) 123.8 g/100 ml (100 °C) | |
| Solubility | slightly soluble in pyridine, soluble in ethanol insoluble in ether |
| +14,350·10−6 cm3/mol | |
| Structure | |
| CdCl2 | |
| octahedral | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
250-275 mg/kg (rat, oral)[citation needed] 1715 mg/kg (mouse, oral)[1] |
| Related compounds | |
Other anions
|
Manganese(II) fluoride Manganese(II) bromide Manganese(II) iodide |
Other cations
|
Manganese(III) chloride Technetium(IV) chloride Rhenium(III) chloride Rhenium(IV) chloride Rhenium(V) chloride Rhenium(VI) chloride |
Related compounds
|
Chromium(II) chloride Iron(II) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Manganese(II) chloride is the dichloride salt of manganese, MnCl2. This inorganic chemical exists in the anhydrous form, as well as the dihydrate (MnCl2·2H2O) and tetrahydrate (MnCl2·4H2O), with the tetrahydrate being the most common form. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d5 configurations.[2]
- ^ "Manganese compounds (as Mn)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
