
Back مركابتوبورين Arabic مرکاپتوپورین AZB Mercaptopurina Catalan Mercaptopwrin Welsh Mercaptopurin German Μερκαπτοπουρίνη Greek Merkaptopurino Esperanto Mercaptopurina Spanish مرکاپتوپورین Persian Merkaptopuriini Finnish
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Purinethol, Purixan, others |
| Other names | 6-mercaptopurine (6-MP) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682653 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 5 to 37% |
| Metabolism | xanthine oxidase |
| Elimination half-life | 60 to 120 min., longer for its active metabolites |
| Excretion | kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.035 |
| Chemical and physical data | |
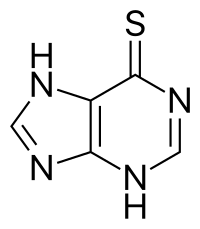
| Formula | C5H4N4S |
| Molar mass | 152.18 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mercaptopurine (6-MP), sold under the brand name Purinethol among others, is a medication used for cancer and autoimmune diseases.[2] Specifically it is used to treat acute lymphocytic leukemia (ALL), acute promyelocytic leukemia (APL), Crohn's disease, and ulcerative colitis.[2][3] For acute lymphocytic leukemia it is generally used with methotrexate.[2] It is taken orally.[2]
Common side effects include bone marrow suppression, liver toxicity, vomiting, and loss of appetite.[2] Other serious side effects include an increased risk of future cancer and pancreatitis.[2] Those with a genetic deficiency in thiopurine S-methyltransferase are at higher risk of side effects.[2] Use in pregnancy may harm the baby.[2] Mercaptopurine is in the thiopurine and antimetabolite family of medications.[4][3]
Mercaptopurine was approved for medical use in the United States in 1953.[2] It is on the World Health Organization's List of Essential Medicines.[5]
- ^ "Xaluprine EPAR". European Medicines Agency. 30 April 2009. Retrieved 25 June 2024.
- ^ a b c d e f g h i "Mercaptopurine". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ a b British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 590. ISBN 9780857111562.
- ^ Sahasranaman S, Howard D, Roy S (August 2008). "Clinical pharmacology and pharmacogenetics of thiopurines". European Journal of Clinical Pharmacology. 64 (8): 753–67. doi:10.1007/s00228-008-0478-6. PMID 18506437. S2CID 27475772.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.