 | |
| Clinical data | |
|---|---|
| Trade names | Ypozane |
| Other names | TZP-4238; Gestoxarone acetate; 2-Oxachloromadinone acetate; 17α-Acetoxy-6-chloro-2-oxa-6-dehydroprogesterone; 17α-Acetoxy-6-chloro-2-oxapregna-4,6-diene-3,20-dione, Osaterone acetate (JAN JP) |
| Routes of administration | By mouth (tablets) |
| Drug class | Steroidal antiandrogen; Progestogen; Progestin; Progestogen ester |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Osaterone acetate: 90% 15β-Hydroxyosaterone acetate: 80%[3] (Both mainly to albumin)[3] |
| Metabolism | Liver[3] |
| Metabolites | 15β-Hydroxyosaterone acetate[3] |
| Elimination half-life | Dogs: 80 hours to 197 ± 109 hours[3][4] |
| Excretion | Bile: 60%[3] Urine: 25%[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.750 |
| Chemical and physical data | |
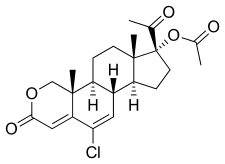
| Formula | C22H27ClO5 |
| Molar mass | 406.90 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Osaterone acetate, sold under the brand name Ypozane, is a medication which is used in veterinary medicine for the treatment of enlarged prostate in dogs.[3][5][6] It is given by mouth.[3]
Osaterone acetate is an antiandrogen, and hence is an antagonist of the androgen receptor, the biological target of androgens like testosterone and dihydrotestosterone.[3] It is also a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[3]
Osaterone acetate was introduced for veterinary use in 2007.[1][3][7][8]
- ^ a b "Ypozane EPAR". European Medicines Agency. 18 January 2007. Retrieved 28 June 2024.
- ^ "Ypozane PI". Union Register of veterinary medicinal products. 15 January 2007. Retrieved 29 June 2024.
- ^ a b c d e f g h i j k l "Ypozane for Dogs" (PDF). European Medicines Agency. Archived from the original (PDF) on 20 June 2018. Retrieved 20 February 2018.
- ^ Cite error: The named reference
MaddisonPage2008was invoked but never defined (see the help page). - ^ Weber GF (22 July 2015). Molecular Therapies of Cancer. Springer. pp. 316–. ISBN 978-3-319-13278-5.
- ^ Greer ML (18 December 2014). Canine Reproduction and Neonatology. Teton NewMedia. pp. 296–. ISBN 978-1-4987-2850-8.
- ^ Cite error: The named reference
EmmerichUngemach2008was invoked but never defined (see the help page). - ^ Cite error: The named reference
Drugs.comwas invoked but never defined (see the help page).
