
Back Dominio PAS Spanish Domaine PAS French PAS domen Serbo-Croatian PAS domen Serbian PAS结构域 Chinese
| PAS fold | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
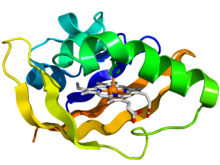 Crystallographic structure of the PAS domain of the bacterial oxygen sensor protein fixL.[1] The protein is depicted as a rainbow colored cartoon (N-terminus = blue, C-terminus = red) while the heme ligand is shown as sticks (carbon = white, nitrogen = blue, oxygen = red, iron = orange). | |||||||||||
| Identifiers | |||||||||||
| Symbol | PAS | ||||||||||
| Pfam | PF00989 | ||||||||||
| Pfam clan | CL0183 | ||||||||||
| InterPro | IPR013767 | ||||||||||
| SMART | PAS | ||||||||||
| PROSITE | PDOC50112 | ||||||||||
| SCOP2 | 2phy / SCOPe / SUPFAM | ||||||||||
| CDD | cd00130 | ||||||||||
| |||||||||||
A Per-Arnt-Sim (PAS) domain is a protein domain found in all kingdoms of life.[2] Generally, the PAS domain acts as a molecular sensor, whereby small molecules and other proteins associate via binding of the PAS domain.[3][4][5] Due to this sensing capability, the PAS domain has been shown as the key structural motif involved in protein-protein interactions of the circadian clock, and it is also a common motif found in signaling proteins, where it functions as a signaling sensor.[6][7]
- ^ PDB: 1y28; Dunham CM, Dioum EM, Tuckerman JR, Gonzalez G, Scott WG, Gilles-Gonzalez MA (July 2003). "A distal arginine in oxygen-sensing heme-PAS domains is essential to ligand binding, signal transduction, and structure". Biochemistry. 42 (25): 7701–8. doi:10.1021/bi0343370. PMID 12820879. S2CID 14090693.
- ^ Henry, Jonathan T.; Crosson, Sean (1 January 2011). "Ligand-binding PAS domains in a genomic, cellular, and structural context". Annual Review of Microbiology. 65: 261–286. doi:10.1146/annurev-micro-121809-151631. PMC 3298442. PMID 21663441.
- ^ Liu, Yu C.; Machuca, Mayra A.; Beckham, Simone A.; Gunzburg, Menachem J.; Roujeinikova, Anna (1 October 2015). "Structural basis for amino-acid recognition and transmembrane signalling by tandem Per-Arnt-Sim (tandem PAS) chemoreceptor sensory domains". Acta Crystallographica Section D. 71 (10): 2127–2136. Bibcode:2015AcCrD..71.2127L. doi:10.1107/S139900471501384X. PMID 26457436.
- ^ Möglich, Andreas; Ayers, Rebecca A.; Moffat, Keith (14 October 2009). "Structure and signaling mechanism of Per-ARNT-Sim domains". Structure. 17 (10): 1282–1294. doi:10.1016/j.str.2009.08.011. PMC 3092527. PMID 19836329.
- ^ Hennig, Sven; Strauss, Holger M.; Vanselow, Katja; Yildiz, Özkan; Schulze, Sabrina; Arens, Julia; Kramer, Achim; Wolf, Eva (28 April 2009). "Structural and Functional Analyses of PAS Domain Interactions of the Clock Proteins Drosophila PERIOD and Mouse PERIOD2". PLOS Biology. 7 (4): e1000094. doi:10.1371/journal.pbio.1000094. PMC 2671562. PMID 19402751.
- ^ Ponting CP, Aravind L (November 1997). "PAS: a multi-functional domain family comes to light". Curr. Biol. 7 (11): R674–7. doi:10.1016/S0960-9822(06)00352-6. PMID 9382818. S2CID 14105830.
- ^ Hefti MH, Françoijs KJ, de Vries SC, Dixon R, Vervoort J (March 2004). "The PAS fold. A redefinition of the PAS domain based upon structural prediction". Eur. J. Biochem. 271 (6): 1198–208. doi:10.1111/j.1432-1033.2004.04023.x. PMID 15009198.