Back فلوريد البيركلوريل Arabic پرکولوریل فلوئورید AZB Perchlorylfluorid German پرکلریل فلوئورید Persian Perkloryylifluoridi Finnish Fluorure de perchloryle French परक्लोरिल फ्लोराइड Hindi Perchlorylfluoride Dutch Перхлорилфторид Russian Perhloril fluorid Serbo-Croatian
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Perchloryl fluoride
| |||
| Other names
Chlorine oxyfluoride, Perchlorofluoride, Chlorine fluorine oxide, Trioxychlorofluoride, Perchloric acid fluoride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.028.660 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
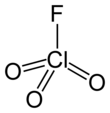
| ClO3F | |||
| Molar mass | 102.4496 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | sweet odor | ||
| Density | 1.434 g/cm3 | ||
| Melting point | −147.8 °C (−234.0 °F; 125.3 K) | ||
| Boiling point | −46.7 °C (−52.1 °F; 226.5 K) | ||
| Critical point (T, P) | 95.2 °C (203.4 °F; 368.3 K), 53 standard atmospheres (5,400 kPa; 780 psi)[1] | ||
| 0.06 g/100 ml (20 °C), slow hydrolysis | |||
| Vapor pressure | 10.5 atm (20 °C)[2] | ||
| Viscosity | 3.91 x 10−3 Pa.s (@ melting point) | ||
| Structure | |||
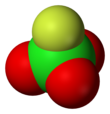
| Tetrahedral[3]: 373 | |||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
278.97 J/(mol*K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
-21.42 kJ/mol [4]: 380 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
oxidizing, toxic. Non-corrosive. | ||
| NFPA 704 (fire diamond) | |||
Threshold limit value (TLV)
|
3 ppm | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
385 ppm (rat, 4 hr) 451 ppm (dog, 4 hr)[5] | ||
LCLo (lowest published)
|
2000 ppm (rat, 40 min) 451 ppm (dog, 4 hr)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 3 ppm (13.5 mg/m3)[2] | ||
REL (Recommended)
|
TWA 3 ppm (14 mg/m3) ST 6 ppm (28 mg/m3)[2] | ||
IDLH (Immediate danger)
|
100 ppm[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Perchloryl fluoride[6] is a reactive gas with the chemical formula ClO
3F. It has a characteristic sweet odor[7] that resembles gasoline and kerosene. It is toxic and is a powerful oxidizing and fluorinating agent. It is the acid fluoride of perchloric acid.
In spite of its small enthalpy of formation (ΔfH° = −5.2 kcal/mol (−22 kJ/mol)), it is kinetically stable, decomposing only at 400 °C.[3]: 380 It is quite reactive towards reducing agents and anions, however, with the chlorine atom acting as an electrophile.[3]: 382 It reacts explosively with reducing agents such as metal amides, metals, hydrides, etc.[7] Its hydrolysis in water occurs very slowly, unlike that of chloryl fluoride.
- ^ Budavari, Susan, ed. (1989). "7297. Perchloryl Fluoride". The Merck Index — Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, New Jersey: Merck. p. 1231. IA147021.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0490". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c Cite error: The named reference
emeleus1976was invoked but never defined (see the help page). - ^ Chase, M. W. (2018). "Perchloryl fluoride". NIST Chemistry WebBook, SRD 69. pp. 1–1951.
- ^ a b "Perchloryl fluoride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Chemical Science and Technology Laboratory. "Perchloryl fluoride". National Institute of Standards and Technology. Retrieved 2009-11-28.
- ^ a b Jared Ledgard (2007). The Preparatory Manual of Explosives (3rd ed.). Lulu.com. p. 77. ISBN 978-0-615-14290-6.