
Back Poliëtileen Afrikaans متعدد الإيثيلين Arabic Polietilen Azerbaijani پولیاتیلن AZB Поліэтылен BE-X-OLD Полиетилен Bulgarian পলিথিন Bengali/Bangla Polietilen BS Polietilè Catalan Polyethylen Czech

| |

| |
| Names | |
|---|---|
| IUPAC name
Polyethene or poly(methylene)[1]
| |
| Other names
Polyethylene
Polythene | |
| Identifiers | |
| Abbreviations | PE |
| ChemSpider |
|
| ECHA InfoCard | 100.121.698 |
| KEGG | |
| MeSH | Polyethylene |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| (C2H4)n | |
| Density | 0.88–0.96 g/cm3[2] |
| Melting point | 115–135 °C (239–275 °F; 388–408 K)[2] |
| Not soluble | |
| log P | 1.02620[3] |
| −9.67×10−6 (HDPE, SI, 22 °C)[4] | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−28 to −29 kJ/mole[5] |
| 650-651 kJ/mole, 46 MJ/kg[5] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Part of a series on |
| Fiber |
|---|
 |
| Natural fibers |
| Human-made fibers |
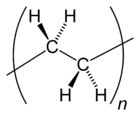
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic.[7] It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, cups, jars, etc.). As of 2017[update], over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.[8][9]
Many kinds of polyethylene are known, with most having the chemical formula (C2H4)n. PE is usually a mixture of similar polymers of ethylene, with various values of n. It can be low-density or high-density and many variations thereof. Its properties can be modified further by crosslinking or copolymerization. All forms are nontoxic as well as chemically resilient, contributing to polyethylene's popularity as a multi-use plastic. However, polyethylene's chemical resilience also makes it a long-lived and decomposition-resistant pollutant when disposed of improperly.[10] Being a hydrocarbon, polyethylene is colorless to opaque (without impurities or colorants) and combustible.[11]
- ^ Compendium of Polymer Terminology and Nomenclature – IUPAC Recommendations 2008 (PDF). Retrieved 28 August 2018.
- ^ a b Batra, Kamal (2014). Role of Additives in Linear Low Density Polyethylene (LLDPE) Films. p. 9. Retrieved 16 September 2014.
- ^ "poly(ethylene)". ChemSrc.
- ^ Wapler, M. C.; Leupold, J.; Dragonu, I.; von Elverfeldt, D.; Zaitsev, M.; Wallrabe, U. (2014). "Magnetic properties of materials for MR engineering, micro-MR and beyond". JMR. 242: 233–242. arXiv:1403.4760. Bibcode:2014JMagR.242..233W. doi:10.1016/j.jmr.2014.02.005. PMID 24705364. S2CID 11545416.
- ^ a b Paul L. Splitstone and Walter H. Johnson (20 May 1974). "The Enthalpies of Combustion and Formation of Linear Polyethylene" (PDF). Journal of Research of the National Bureau of Standards.
- ^ Hemakumara, G. P. T. S.; Madhusankha, T. G. Shamal (2023). "Challenges of Reducing Polythene and Plastic in Sri Lanka: A Case Study of Attanagalla Secretariat Division". Socially Responsible Plastic. Developments in Corporate Governance and Responsibility. 19: 59–73. doi:10.1108/S2043-052320230000019004. ISBN 978-1-80455-987-1.
- ^ Cite error: The named reference
Ullmannwas invoked but never defined (see the help page). - ^ Geyer, Roland; Jambeck, Jenna R.; Law, Kara Lavender (1 July 2017). "Production, use, and fate of all plastics ever made". Science Advances. 3 (7): e1700782. Bibcode:2017SciA....3E0782G. doi:10.1126/sciadv.1700782. PMC 5517107. PMID 28776036.
- ^ "Plastics: The Facts" (PDF). Plastics Europe. Archived from the original (PDF) on 4 February 2018. Retrieved 29 August 2018.
- ^ Yao, Zhuang; Jeong Seong, Hyeon; Jang, Yu-Sin (2022). "Environmental toxicity and decomposition of polyethylene". Ecotoxicology and Environmental Safety. 242: 1, 3. Bibcode:2022EcoES.24213933Y. doi:10.1016/j.ecoenv.2022.113933. PMID 35930840.
- ^ Sepe, Michael (8 April 2024). "Understanding the 'Science' of Color". Plastics Technology. Retrieved 25 April 2024.