
Back أميد البوتاسيوم Arabic Amid draselný Czech Kaliumamid German Kaliamido Esperanto Amida de potasio Spanish Amidure de potassium French カリウムアミド Japanese പൊട്ടാസ്യം അമൈഡ് Malayalam Амид калия Russian Kalijum amid Serbo-Croatian
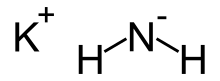
| |

| |
| Names | |
|---|---|
| IUPAC name
Potassium amide
| |
| Other names
Potassamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.508 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| KNH2 | |
| Molar mass | 55.121 g·mol−1 |
| Appearance | white solid |
| Odor | ammonia-like |
| Density | 1.57 g/cm 3 |
| Melting point | 338 °C (640 °F; 611 K) |
| reacts | |
| Solubility | ammonia: 3.6 g/(100 mL) |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-128.9 kJ/mol |
| Related compounds | |
Other cations
|
Lithium amide Sodium amide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium amide is an inorganic compound with the chemical formula KNH2. Like other alkali metal amides, it is a white solid that hydrolyzes readily. It is a strong base.[1]
- ^ Takaki, Katherine S. (2001). "Potassium Amide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rp193. ISBN 0471936235.