
Back سولفات پرازئودیومیوم AZB Síran praseodymitý Czech Praseodym(III)-sulfat German سولفات پرازئودیمیم Persian Sulfat de praseodim Romanian Сульфат празеодима(III) Russian Prazeodijum(III) sulfat Serbo-Croatian Prazeodijum(III) sulfat Serbian Празеодим(ІІІ) сульфат Ukrainian Praseodymi(III) sulfat Vietnamese
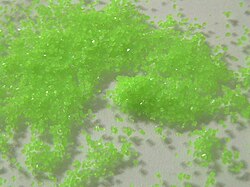 Praseodymium sulfate octahydrate
| |
| Names | |
|---|---|
| IUPAC name
praseodymium(3+); trisulfate
| |
| Other names
Praseodymium sulphate, dipraseodymium trisulphate, praseodymium(III) sulfate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.553 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Pr2(SO4)3 Pr2(SO4)3·nH2O, n=2,5,8 | |
| Molar mass | 570.0031 g/mol 714.12534 g/mol (octahydrate) |
| Appearance | green crystalline solid |
| Density | 3.72 g/cm3[1] |
| Melting point | 1,010 °C (1,850 °F; 1,280 K) (decomposes)[1] |
| 113.0 g/L (20 °C) 108.8 g/L (25 °C) | |
| +9660·10−6 cm3/mol | |
| Hazards | |
| GHS labelling:[2] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Praseodymium carbonate Praseodymium chloride |
Other cations
|
Neodymium sulfate |
Related compounds
|
Praseodymium(III) oxide Praseodymium(III) sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Praseodymium(III) sulfate is a praseodymium compound with formula Pr2(SO4)3. It is an odourless whitish-green crystalline compound. The anhydrous substance readily absorbs water forming pentahydrate and octahydrate.[1]