
Back برافاستاتين Arabic پروستاتین AZB Prafastatin Welsh Pravastatin German Pravastatina Spanish پراواستاتین Persian Pravastatine French Pravasztatin Hungarian Pravastatina Italian Pravastatine Dutch
 | |
| Clinical data | |
|---|---|
| Trade names | Pravachol, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692025 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 18%[4] |
| Protein binding | 50%[4] |
| Metabolism | Liver (minimal)[4] |
| Elimination half-life | 1-3 hours[4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.216.225 |
| Chemical and physical data | |
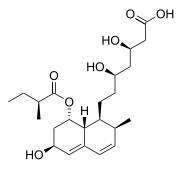
| Formula | C23H36O7 |
| Molar mass | 424.534 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pravastatin, sold under the brand name Pravachol among others, is a statin medication, used for preventing cardiovascular disease in those at high risk and treating abnormal lipids.[5] It is suggested to be used together with diet changes, exercise, and weight loss.[5] It is taken by mouth.[5]
Common side effects include joint pain, diarrhea, nausea, headaches, and muscle pains.[5] Serious side effects may include rhabdomyolysis, liver problems, and diabetes.[5] Use during pregnancy may harm the fetus.[5] Like all statins, pravastatin works by inhibiting HMG-CoA reductase, an enzyme found in liver that plays a role in producing cholesterol.[5]
Pravastatin was patented in 1980 and approved for medical use in 1989.[6] It is on the World Health Organization's List of Essential Medicines.[7] It is available as a generic medication.[5] In 2022, it was the 37th most commonly prescribed medication in the United States, with more than 16 million prescriptions.[8][9]
- ^ Cite error: The named reference
Pravastatin FDA labelwas invoked but never defined (see the help page). - ^ "Pravachol (pravastatin sodium) Tablets Initial U.S. Approval: 1991". DailyMed. Retrieved 2 September 2024.
- ^ "Active substance: pravastatin" (PDF). List of nationally authorised medicinal products. European Medicines Agency. 26 November 2020.
- ^ a b c d Neuvonen PJ, Backman JT, Niemi M (2008). "Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin". Clinical Pharmacokinetics. 47 (7): 463–474. doi:10.2165/00003088-200847070-00003. PMID 18563955. S2CID 11716425.
- ^ a b c d e f g h "Pravastatin Sodium Monograph for Professionals". Drugs.com. AHFS. Retrieved 23 December 2018.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 472. ISBN 9783527607495.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Pravastatin Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.