
Back پرینول AZB Prenol German Prenolo Esperanto Prenool Estonian پرینول Persian Prenoli Finnish Prénol French Prenol Galician Prenolo Italian プレノール Japanese
| |
| |
| Names | |
|---|---|
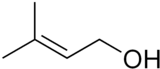
| Preferred IUPAC name
3-Methylbut-2-en-1-ol | |
| Other names
3,3-Dimethylallyl alcohol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.312 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[1] | |
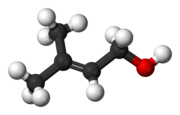
| C5H10O | |
| Molar mass | 86.132 g/mol |
| Density | 0.848 g/cm3 |
| Melting point | −59 °C (−74 °F; 214 K) (calculated) |
| Boiling point | 142 °C (288 °F; 415 K) (approximation) |
| 17 g/100 ml (20 °C) | |
| log P | 0.91 |
| Vapor pressure | 3.17 hPa (25 °C, extrapolated) |
| Hazards[1][2] | |
| GHS labelling: | |
 
| |
| Warning | |
| H226, H302 | |
| P210, P233, P240, P241, P242, P243, P264, P270, P301+P312, P303+P361+P353, P330, P370+P378, P403+P235, P501 | |
| Flash point | 43.3 °C (109.9 °F; 316.4 K)[note 1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Prenol, or 3-methyl-2-buten-1-ol, is a natural alcohol. It is one of the most simple terpenoids. It is a clear colorless oil that is reasonably soluble in water and miscible with most common organic solvents. It has a fruity odor and is used occasionally in perfumery.
Prenol occurs naturally in citrus fruits, cranberry, bilberry, currants, grapes, raspberry, blackberry, tomato, white bread, hop oil, coffee, arctic bramble, cloudberry and passion fruit.[1] It is also manufactured industrially by BASF (in Ludwigshafen, Germany) and by Kuraray (in Asia) as an intermediate to pharmaceuticals and aroma compounds. Global production in 2001 was between 6000 and 13,000 tons.[1]
- ^ a b c d 3-Methyl-2-buten-1-ol (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, May 2005.
- ^ HSNO Chemical Classification Information Database, New Zealand Environmental Risk Management Agency, retrieved 2009-08-31[permanent dead link].
Cite error: There are <ref group=note> tags on this page, but the references will not show without a {{reflist|group=note}} template (see the help page).