
Back سودوإفيدرين Arabic Psevdoefedrin Azerbaijani سودوافدرین AZB সিউডোফেড্রিন Bengali/Bangla Pseudoefedrina Catalan Pseudoefedrin Czech Ffugeffedrin Welsh Pseudoephedrin German Pseudoefedrina Spanish سودوافدرین Persian
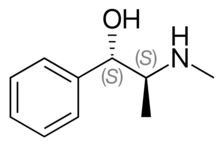 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌsuːdoʊ.ɪˈfɛdrɪn, -ˈɛfɪdriːn/ |
| Trade names | Afrinol, Sudafed, Sinutab, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682619 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~100%[6] |
| Metabolism | 10–30% liver |
| Elimination half-life | 4.3–8 hours[6] |
| Excretion | 43–96% kidney[6] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.835 |
| Chemical and physical data | |
| Formula | C10H15NO |
| Molar mass | 165.236 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Pseudoephedrine (PSE) is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It may be used as a nasal/sinus decongestant,[7] as a stimulant, or as a wakefulness-promoting agent[8] in higher doses.[9]
It was first characterized in 1889 by the German chemists Ladenburg and Oelschlägel, who used a sample that had been isolated from Ephedra vulgaris by the Merck pharmaceutical corporation of Darmstadt, Germany.[10] The salts pseudoephedrine hydrochloride and pseudoephedrine sulfate are found in over-the-counter preparations, either as a single ingredient or (more commonly) in a fixed-dose combination with one or more additional active ingredients such as antihistamines, guaifenesin, dextromethorphan, paracetamol (acetaminophen), or an NSAID (such as aspirin or ibuprofen).
- ^ "Project STOP A Pharmacy Guild Initiative, May 2016" (PDF). The Pharmacy Guild of Australia. 18 May 2016.
{{cite web}}: CS1 maint: url-status (link) - ^ "Trends & issues in crime and criminal justice No. 509, March 2016" (PDF). Australian Institute of Criminology. 7 March 2016. Retrieved 11 July 2024.
{{cite web}}: CS1 maint: url-status (link) - ^ "Project STOP mandatory for pharmacists in NSW from next month". Pulse.IT. 24 February 2016. Retrieved 11 July 2024.
{{cite web}}: CS1 maint: url-status (link) - ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 15 August 2023.
- ^ "Background on Update to NAPRA NHP Policy". napra.ca. 10 June 2024. Retrieved 11 July 2024.
{{cite web}}: CS1 maint: url-status (link) - ^ a b c Brunton LL, Lazo JS, Parker K, eds. (2006). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill Medical Publishing Division. ISBN 0-07-142280-3.
- ^ Al-Ahmad M, Hassab M, Al Ansari A (21 December 2020). "Allergic and Non-allergic Rhinitis". Textbook of Clinical Otolaryngology. Cham: Springer International Publishing. pp. 241–252. doi:10.1007/978-3-030-54088-3_22. ISBN 978-3-030-54087-6. S2CID 234142758.
Oral decongestants such as pseudoephedrine combined with an antihistamine and/or INC sprays are used in patients with nasal congestion.
- ^ Hodges K, Hancock S, Currell K, Hamilton B, Jeukendrup AE (February 2006). "Pseudoephedrine enhances performance in 1500-m runners". Medicine and Science in Sports and Exercise. 38 (2): 329–333. doi:10.1249/01.mss.0000183201.79330.9c. PMID 16531903.
- ^ Trinh KV, Kim J, Ritsma A (November 2015). "Effect of pseudoephedrine in sport: a systematic review". BMJ Open Sport & Exercise Medicine. 1 (1): e000066. doi:10.1136/bmjsem-2015-000066. PMC 5117033. PMID 27900142.
- ^ Ladenburg A, Oelschlägel C (1889). "Ueber das "Pseudo-Ephedrin"" [On pseudo-ephedrine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 22 (2): 1823–1827. doi:10.1002/cber.18890220225. Archived from the original on 8 March 2021. Retrieved 19 May 2019.