
Back Straling Afrikaans Strahlung ALS ጨረራ Amharic إشعاع Arabic إشعاع ARY Şüalanma Azerbaijani Выпраменьванне Byelorussian Выпраменьваньне BE-X-OLD Лъчение Bulgarian বিকিরণ Bengali/Bangla

In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium.[1][2] This includes:
- electromagnetic radiation consisting of photons, such as radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, and gamma radiation (γ)
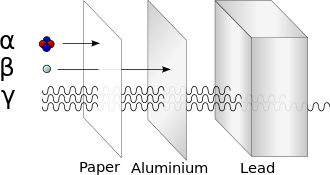
- particle radiation consisting of particles of non-zero rest energy, such as alpha radiation (α), beta radiation (β), proton radiation and neutron radiation
- acoustic radiation, such as ultrasound, sound, and seismic waves, all dependent on a physical transmission medium
- gravitational radiation, in the form of gravitational waves, ripples in spacetime
Radiation is often categorized as either ionizing or non-ionizing depending on the energy of the radiated particles. Ionizing radiation carries more than 10 electron volts (eV), which is enough to ionize atoms and molecules and break chemical bonds. This is an important distinction due to the large difference in harmfulness to living organisms. A common source of ionizing radiation is radioactive materials that emit α, β, or γ radiation, consisting of helium nuclei, electrons or positrons, and photons, respectively. Other sources include X-rays from medical radiography examinations and muons, mesons, positrons, neutrons and other particles that constitute the secondary cosmic rays that are produced after primary cosmic rays interact with Earth's atmosphere.
Gamma rays, X-rays, and the higher energy range of ultraviolet light constitute the ionizing part of the electromagnetic spectrum. The word "ionize" refers to the breaking of one or more electrons away from an atom, an action that requires the relatively high energies that these electromagnetic waves supply. Further down the spectrum, the non-ionizing lower energies of the lower ultraviolet spectrum cannot ionize atoms, but can disrupt the inter-atomic bonds that form molecules, thereby breaking down molecules rather than atoms; a good example of this is sunburn caused by long-wavelength solar ultraviolet. The waves of longer wavelength than UV in visible light, infrared, and microwave frequencies cannot break bonds but can cause vibrations in the bonds which are sensed as heat. Radio wavelengths and below generally are not regarded as harmful to biological systems. These are not sharp delineations of the energies; there is some overlap in the effects of specific frequencies.[3]
The word "radiation" arises from the phenomenon of waves radiating (i.e., traveling outward in all directions) from a source. This aspect leads to a system of measurements and physical units that apply to all types of radiation. Because such radiation expands as it passes through space, and as its energy is conserved (in vacuum), the intensity of all types of radiation from a point source follows an inverse-square law in relation to the distance from its source. Like any ideal law, the inverse-square law approximates a measured radiation intensity to the extent that the source approximates a geometric point.
- ^ Weisstein, Eric W. "Radiation". Eric Weisstein's World of Physics. Wolfram Research. Retrieved 11 January 2014.
- ^ "Radiation". The free dictionary by Farlex. Farlex, Inc. Retrieved 11 January 2014.
- ^ "The Electromagnetic Spectrum". Centers for Disease Control and Prevention. 7 December 2015. Retrieved 29 August 2018.