Back Seewater Afrikaans ماء البحر Arabic Dəniz suyu Azerbaijani Морска вода Bulgarian खारा पानी Bihari Solwota Bislama সমুদ্রের পানি Bengali/Bangla Morska voda BS Aigua de mar Catalan ئاوی دەریا CKB
| Part of a series on |
| Water salinity |
|---|
 |
| Salinity levels |
|
Fresh water (< 0.05%) Brackish water (0.05–3%) Saline water (3–5%) Brine (> 5% up to 26%–28% max) |
| Bodies of water |
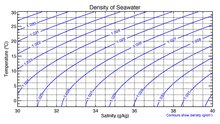
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately 35 grams (1.2 oz) of dissolved salts (predominantly sodium (Na+
) and chloride (Cl−
) ions). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water and pure water (density 1.0 kg/L at 4 °C (39 °F)) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about −2 °C (28 °F).[1] The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was −2.6 °C (27.3 °F).[2]
Seawater pH is typically limited to a range between 7.5 and 8.4.[3] However, there is no universally accepted reference pH-scale for seawater and the difference between measurements based on different reference scales may be up to 0.14 units.[4]
- ^ "U.S. Office of Naval Research Ocean, Water: Temperature". Archived from the original on 12 December 2007.
- ^ Sylte, Gudrun Urd (24 May 2010). "Den aller kaldaste havstraumen". forskning.no (in Norwegian). Archived from the original on 6 March 2012. Retrieved 24 May 2010.
- ^ Chester, Jickells, Roy, Tim (2012). Marine Geochemistry. Blackwell Publishing. ISBN 978-1-118-34907-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Stumm, W, Morgan, J. J. (1981) Aquatic Chemistry, An Introduction Emphasizing Chemical Equilibria in Natural Waters. John Wiley & Sons. pp. 414–416. ISBN 0471048313.