
Back Fluorid stříbřitý Czech Fluoruro de plata(III) Spanish Srebro(III) fluorid Serbian வெள்ளி(III) புளோரைடு Tamil 三氟化银 Chinese
| |
| Names | |
|---|---|
| IUPAC name
Silver(III) fluoride
| |
| Other names
Silver trifluoride
Argentic fluoride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 100808 | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| AgF3 | |
| Molar mass | 164.86 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
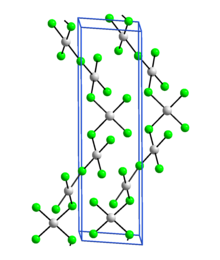
Silver(III) fluoride, AgF3, is an unstable, bright-red, diamagnetic compound containing silver in the unusual +3 oxidation state. Its crystal structure is very similar to that of gold(III) fluoride: it is a polymer consisting of rectangular AgF4 units linked into chains by fluoro bridges.[1]
- ^ Zemva, Boris; Lutar, Karel; Jesih, Adolf; Casteel, William J.; Wilkinson, Angus P.; Cox, David E.; von Dreele, Robert B.; Borrmann, Horst; Bartlett, Neil (1991). "Silver Trifluoride: Preparation, Crystal Structure, Some Properties, and Comparison with AuF3". J Am Chem Soc. 113 (11): 4192–4198. doi:10.1021/ja00011a021.