
Back سيمفاستاتين Arabic سیمواستاتین AZB Simvastatina Catalan Simfastatin Welsh Simvastatin Danish Simvastatin German Σιμβαστατίνη Greek Simvastatina Spanish Sinbastatina Basque سیمواستاتین Persian
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈsɪmvəstætɪn/ |
| Trade names | Zocor, other |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692030 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 5% |
| Protein binding | 95% |
| Metabolism | Liver (CYP3A4) |
| Elimination half-life | 2 hours for simvastatin and 1.9 hours for simvastatin acid |
| Excretion | Kidney 13%, faecal 60% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.115.749 |
| Chemical and physical data | |
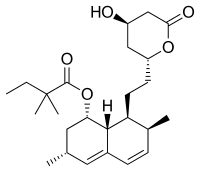
| Formula | C25H38O5 |
| Molar mass | 418.574 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Simvastatin, sold under the brand name Zocor among others, is a statin, a type of lipid-lowering medication.[4] It is used along with exercise, diet, and weight loss to decrease elevated lipid levels.[4] It is also used to decrease the risk of heart problems in those at high risk.[4] It is taken by mouth.[4]
Common side effects include constipation, headaches, and nausea.[4] Serious side effects may include muscle breakdown, liver problems, and increased blood sugar levels.[4] A lower dose may be needed in people with kidney problems.[4] There is evidence of harm to the developing baby when taken during pregnancy[4][5] and it should not be used by those who are breastfeeding.[4] It is in the statin class of medications and works by decreasing the manufacture of cholesterol by the liver.[4]
Simvastatin is made from the fungus Aspergillus terreus.[6] It was patented by Merck in 1980, and came into medical use in 1992.[6][7] Simvastatin is available as a generic medication,[4] and is on the World Health Organization's List of Essential Medicines.[8] In 2022, it was the nineteenth most commonly prescribed medication in the United States, with more than 26 million prescriptions.[9][10]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Cite error: The named reference
Zocor FDA labelwas invoked but never defined (see the help page). - ^ "Active substance: simvastatin" (PDF). List of nationally authorised medicinal products. European Medicines Agency. 26 November 2020.
- ^ a b c d e f g h i j k "Simvastatin". The American Society of Health-System Pharmacists. Archived from the original on 10 January 2015. Retrieved 8 January 2015.
- ^ "Prescribing medicines in pregnancy database". Australian Government. 3 March 2014. Archived from the original on 8 April 2014. Retrieved 22 April 2014.
- ^ a b Cechinel-Filho V (2012). Plant bioactives and drug discovery : principles, practice, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 104. ISBN 9780470582268. Archived from the original on 5 March 2016.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 472. ISBN 9783527607495.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Simvastatin Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.