
Back ثنائي ثيونيت الصوديوم Arabic جوهر قند AZB Ditionit de sodi Catalan Dithioničitan sodný Czech Natriumdithionit German Natria dutionito Esperanto Ditionito de sodio Spanish سدیم دیتیونیت Persian Natriumditioniitti Finnish Dithionite de sodium French
| |

| |

| |
| Names | |
|---|---|
| Other names
D-Ox, Hydrolin, Reductone
sodium hydrosulfite, sodium sulfoxylate, Sulfoxylate Vatrolite, Virtex L Hydrosulfit, Prayon Blankit, Albite A, Konite Zepar, Burmol, Arostit | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.991 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1384 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
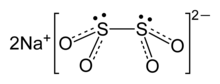
| Na2S2O4 | |
| Molar mass | 174.107 g/mol (anhydrous) 210.146 g/mol (dihydrate) |
| Appearance | white to grayish crystalline powder light-lemon colored flakes |
| Odor | faint sulfur odor |
| Density | 2.38 g/cm3 (anhydrous) 1.58 g/cm3 (dihydrate) |
| Melting point | 52 °C (126 °F; 325 K) |
| Boiling point | Decomposes |
| 18.2 g/100 mL (anhydrous, 20 °C) 21.9 g/100 mL (Dihydrate, 20 °C) | |
| Solubility | slightly soluble in alcohol |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H251, H302 | |
| P235+P410, P264, P270, P280, P301+P312, P330, P407, P413, P420, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 100 °C (212 °F; 373 K) |
| 200 °C (392 °F; 473 K) | |
| Related compounds | |
Other anions
|
Sodium sulfite Sodium sulfate |
Related compounds
|
Sodium thiosulfate Sodium bisulfite Sodium metabisulfite Sodium bisulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium dithionite (also known as sodium hydrosulfite) is a white crystalline powder with a sulfurous odor. Although it is stable in dry air, it decomposes in hot water and in acid solutions.
