
Back سداسي نتروكوبالتات الصوديوم Arabic کوبالتینیتریت سودیوم AZB Hexanitrokobaltitan sodný Czech Natriumhexanitrocobaltat(III) German Natria kobaltonitrito Esperanto کوبالتینیتریت سدیم Persian सोडियम कोबाल्टनाइट्राइट Hindi Նատրիումի հեքսանիտրոկոբալտատ(III) Armenian Esanitrocobaltato di sodio Italian ヘキサニトロコバルト(III)酸ナトリウム Japanese
| |

| |
| Names | |
|---|---|
| IUPAC name
Sodium hexanitritocobaltate(III)
| |
| Other names
Sodium cobaltinitrite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.692 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
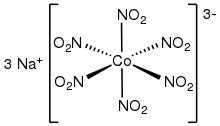
| Na3[Co(NO2)6] | |
| Molar mass | 403.933 g·mol−1 |
| Appearance | Yellow crystals |
| Density | 2.565 g/cm3 |
| Hazards | |
| GHS labelling:[1] | |
  
| |
| Danger | |
| H272, H315, H317, H319, H334, H335, H351 | |
| Safety data sheet (SDS) | JT Baker MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium hexanitritocobaltate(III) is inorganic compound with the formula Na3[Co(NO2)6]. The anion of this yellow-coloured salt consists of the transition metal nitrite complex [Co(NO2)6]3−. It was a reagent for the qualitative test for potassium and ammonium ions.[2]
- ^ "C&L Inventory". echa.europa.eu.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.