
Back هيبوكلوريت الصوديوم Arabic سودیوم هیپوکولوریت AZB Натриев хипохлорит Bulgarian সোডিয়াম হাইপোক্লোরাইট Bengali/Bangla Hipoclorit de sodi Catalan Chlornan sodný Czech Natriumhypoklorit Danish Natriumhypochlorit German Υποχλωριώδες νάτριο Greek Natria hipoklorito Esperanto
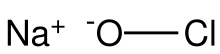
| |
 | |
| Names | |
|---|---|
| IUPAC name
Sodium hypochlorite
| |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.028.790 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1791 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NaOCl | |
| Molar mass | 74.442 g/mol |
| Appearance |
|
| Odor | Chlorine-like and sweetish (pentahydrate)[1] |
| Density | 1.11 g/cm3 |
| Melting point | 18 °C (64 °F; 291 K) (pentahydrate) |
| Boiling point | 101 °C (214 °F; 374 K) (decomposes) (pentahydrate) |
| 29.3 g/(100 mL) (0 °C)[2] | |
| Acidity (pKa) | 7.5185 |
| Basicity (pKb) | 6.4815 |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−347.1 kJ/mol |
| Pharmacology | |
| D08AX07 (WHO) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Oxidizer, corrosive[3] |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H314, H410 | |
| P260, P264, P273, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P391, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | ICSC 1119 (solution, >10% active chlorine) ICSC 0482 (solution, <10% active chlorine) |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium hypochlorite is an alkaline inorganic chemical compound with the formula NaOCl (also written as NaClO). It is commonly known in a dilute aqueous solution as bleach or chlorine bleach.[4] It is the sodium salt of hypochlorous acid, consisting of sodium cations (Na+) and hypochlorite anions (−OCl, also written as OCl− and ClO−).
The anhydrous compound is unstable and may decompose explosively.[5][6] It can be crystallized as a pentahydrate NaOCl·5H2O, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated.[7][8][9]
Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution referred to as chlorine bleach, which is a household chemical widely used (since the 18th century) as a disinfectant and bleaching agent. In solution, the compound is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Sodium hypochlorite is still the most important chlorine-based bleach.[10][11]
Its corrosive properties, common availability, and reaction products make it a significant safety risk. In particular, mixing liquid bleach with other cleaning products, such as acids found in limescale-removing products, will release chlorine gas. Chlorine gas was utilized as a chemical weapon in World War I.[12][13][14] A common misconception is that mixing bleach with ammonia also releases chlorine, but in reality they react to produce chloramines such as nitrogen trichloride. With excess ammonia and sodium hydroxide, hydrazine may be generated.
- ^ a b https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-Hypochlorite
- ^ Budavari S, O'Neil M, Smith A, Heckelman P, Obenchain J (1996). "Sodium hypochlorite". The Merck Index (12th ed.). p. 1478. ISBN 978-0-911910-12-4.
- ^ Sodium hypochlorite: chemical activity
- ^ "sodium hypochlorite | chemical compound | Britannica". www.britannica.com. Retrieved 21 March 2022.
- ^ Cite error: The named reference
bretwas invoked but never defined (see the help page). - ^ Cite error: The named reference
hamano1was invoked but never defined (see the help page). - ^ Cite error: The named reference
applewas invoked but never defined (see the help page). - ^ Cite error: The named reference
okada1was invoked but never defined (see the help page). - ^ Topić F, Marrett JM, Borchers TH, Titi HM, Barrett CJ, Friščić T (2021). "After 200 Years: The Structure of Bleach and Characterization of Hypohalite Ions by Single-Crystal X-Ray Diffraction". Angew. Chem. Int. Ed. 60 (46): 24400–24405. doi:10.1002/anie.202108843. PMID 34293249. S2CID 236199263.
- ^ "OxyChem Sodium Hypochlorite Handbook" (PDF). OxyChem. Archived from the original (PDF) on 18 April 2018. Retrieved 6 February 2015.
- ^ "Pamphlet 96, The Sodium Hypochorite Manual". The Chlorine Institute.
- ^ Faith T (2014). Behind the Gas Mask: The U.S. Chemical Warfare Service in War and Peace. Champaign, Illinois: University of Illinois Press. p. 9. ISBN 978-0252080265. Retrieved 14 April 2017.
- ^ "April 22, 1915: Germans introduce poison gas". This Day In History. Retrieved 14 April 2017.
- ^ Gross DA (Spring 2015). "Chemical Warfare: From the European Battlefield to the American Laboratory". Distillations. 1 (1): 16–23. Retrieved 20 March 2018.
