
Back بطارية صلبة الحالة Arabic সলিড স্টেট ব্যাটারি Bengali/Bangla Bateria d'estat sòlid Catalan Baterie s pevným elektrolytem Czech Festkörperakkumulator German Batería de estado sólido Spanish باتری حالت جامد Persian Batterie solide French סוללת מצב מוצק HE Accumulatore allo stato solido Italian
A solid-state battery is an electrical battery that uses a solid electrolyte for ionic conductions between the electrodes, instead of the liquid or gel polymer electrolytes found in conventional batteries.[1] Solid-state batteries theoretically offer much higher energy density than the typical lithium-ion or lithium polymer batteries.[2]
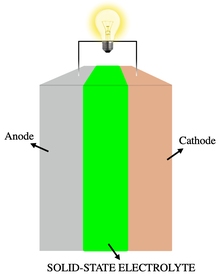 All-solid-state battery with a solid electrolyte between two electrodes | |
| Specific energy | Thin film type: 300–900 Wh/kg (490–1,470 kJ/lb) Bulk type: 250–500 Wh/kg (410–820 kJ/lb) |
|---|---|
| Self-discharge rate | 6%ー85 °C (month) [3] |
| Cycle durability | 10,000-100,000 cycles [3] |
| Nominal cell voltage | Thin film type: 4.6 V[4] Bulk type: 2.5 V, [3] |
| Operating temperature interval | -50 °C 〜 125 °C |
| Charge temperature interval | -20 °C 〜 105 °C |
While solid electrolytes were first discovered in the 19th century, several issues prevented widespread application. Developments in the late 20th and early 21st century generated renewed interest in the technology, especially in the context of electric vehicles.
Solid-state batteries can use metallic lithium for the anode and oxides or sulfides for the cathode, increasing energy density. The solid electrolyte acts as an ideal separator that allows only lithium ions to pass through. For that reason, solid-state batteries can potentially solve many problems of currently used liquid electrolyte Li-ion batteries, such as flammability, limited voltage, unstable solid-electrolyte interface formation, poor cycling performance, and strength.[5]
Materials proposed for use as electrolytes include ceramics (e.g., oxides, sulfides, phosphates), and solid polymers. Solid-state batteries are found in pacemakers, and in RFID and wearable devices. Solid-state batteries are potentially safer, with higher energy densities. Challenges to widespread adoption include energy and power density, durability, material costs, sensitivity, and stability.[6]
- ^ Vandervell, Andy (26 September 2017). "What is a solid-state battery? The benefits explained". Wired UK. Retrieved 7 January 2018.
- ^ Reisch, Marc S. (20 November 2017). "Solid-state batteries inch their way to market". C&EN Global Enterprise. 95 (46): 19–21. doi:10.1021/cen-09546-bus.
- ^ a b c "セラミックパッケージ型全固体電池・評価用電源モジュールキット|二次電池|Biz.maxell - マクセル". Biz.maxell - マクセル.
- ^ "コイン形全固体電池・バイポーラ型全固体電池|二次電池|Biz.maxell - マクセル". Biz.maxell - マクセル.
- ^ Ping, Weiwei; Yang, Chunpeng; Bao, Yinhua; Wang, Chengwei; Xie, Hua; Hitz, Emily; Cheng, Jian; Li, Teng; Hu, Liangbing (September 2019). "A silicon anode for garnet-based all-solid-state batteries: Interfaces and nanomechanics". Energy Storage Materials. 21: 246–252. doi:10.1016/j.ensm.2019.06.024. S2CID 198825492.
- ^ Weppner, Werner (September 2003). "Engineering of solid state ionic devices". International Journal of Ionics. 9 (5–6): 444–464. doi:10.1007/BF02376599. S2CID 108702066.
Solid state ionic devices such as high performance batteries...