
Back Sulfamidlər Azerbaijani سولفامید AZB Sulfamid Czech Sulfamid German سولفامید Persian Sulfamide French Szulfamid Hungarian スルファミド Japanese Сульфамид Russian Sulfamid Serbo-Croatian
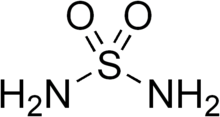
| |

| |
| Names | |
|---|---|
| IUPAC name
Sulfuric diamide
| |
| Preferred IUPAC name
Sulfamide | |
| Other names
Sulphamide
Sulfuryl amide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.029.330 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H4N2O2S | |
| Molar mass | 96.11 g/mol |
| Appearance | White orthorhombic plates |
| Melting point | 93 °C (199 °F; 366 K) |
| Boiling point | 250 °C (482 °F; 523 K) (decomposes) |
| Freely soluble | |
| -44.4×10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sulfamide (IUPAC name: sulfuric diamide) is a compound with the chemical formula SO2(NH2)2 and structure H2N−S(=O)2−NH2. Sulfamide is produced by the reaction of sulfuryl chloride with ammonia. Sulfamide was first prepared in 1838 by the French chemist Henri Victor Regnault.[2]
- ^ Merck Index, 11th Edition, 8894.
- ^ Regnault, Victor (1838) "Sur l'acide chlorosulfurique et la sulfamide" (On sulfuryl chloride and sulfamide), Annales de chimie et de physique, series 2, 69 : 170-184; see especially "Action de gaz ammoniac sec sur la liqueur chlorosulfurique" (Action of dry ammonia gas on liquid sulfuryl chloride), pages 176-180.