
Back Sulfonat BS Sulfonat Catalan Sulfonáty Czech Sylffonad Welsh Sulfonat Danish Sulfonato Spanish سولفونات Persian Sulfonaatti Finnish Sulfonate French Sulfonato Galician
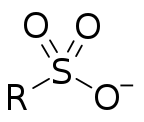
In organosulfur chemistry, a sulfonate is a salt, anion or ester of a sulfonic acid. Its formula is R−S(=O)2−O−, containing the functional group −S(=O)2−O−, where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates.