
Back ثيوإستر Arabic Tioester BS Tioèster Catalan Thioestery Czech Thioester German Tioéster Spanish تیواستر Persian Tioesterit Finnish Thioester French Tioéster Galician
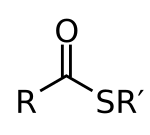
In organic chemistry, thioesters are organosulfur compounds with the molecular structure R−C(=O)−S−R’. They are analogous to carboxylate esters (R−C(=O)−O−R’) with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid (R−C(=O)−O−H) with a thiol (R'−S−H). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.[1] The R and R' represent organyl groups, or H in the case of R.
- ^ Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. doi:10.1002/9780470771099.ch15