
Back فلوريد القصدير الثنائي Arabic فلوئورید تنهکه (II) AZB Fluorid cínatý Czech Zinn(II)-fluorid German Fluoruro de estaño(II) Spanish فلوئورید قلع (II) Persian Tina(II)fluoridi Finnish Fluorure d'étain(II) French टिन(II) फ्लोराइड Hindi Калај(II) флуорид Macedonian
 Sn2+; F−
| |
| Names | |
|---|---|
| IUPAC name
Tin(II) fluoride
| |
| Other names
Stannous fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.029.090 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3288 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SnF2 | |
| Molar mass | 156.69 g/mol |
| Appearance | colorless solid |
| Density | 4.57 g/cm3 |
| Melting point | 213 °C (415 °F; 486 K) |
| Boiling point | 850 °C (1,560 °F; 1,120 K) |
| 31 g/100 mL (0 °C); 35 g/100 mL (20 °C); 78.5 g/100 mL (106 °C) | |
| Solubility | soluble in KOH, KF; negligible in ethanol, ether, chloroform |
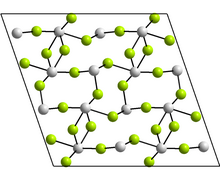
| Structure | |
| Monoclinic, mS48 | |
| C2/c, No. 15 | |
| Pharmacology | |
| A01AA04 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 0860 |
| Related compounds | |
Other anions
|
Tin(II) chloride, Tin(II) bromide, Tin(II) iodide |
Other cations
|
Difluorocarbene, Carbon tetrafluoride, Difluorosilylene, Silicon tetrafluoride, Difluorogermylene, Germanium tetrafluoride, Tin tetrafluoride, Lead(II) fluoride, Lead(IV) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tin(II) fluoride, commonly referred to commercially as stannous fluoride[1][2] (from Latin stannum, 'tin'), is a chemical compound with the formula SnF2. It is a colourless solid used as an ingredient in toothpastes.
- ^ "National Inventors Hall of Fame Announces 2019 Inductees at CES" (Press release). National Inventors Hall of Fame. Retrieved 6 February 2019.
- ^ "Latin Names Variable Charge Metals". Nobel.SCAS.BCIT.ca/. British Columbia Institute of Technology Chemistry Department. Archived from the original on 22 July 2020. Retrieved 16 June 2013.
