 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Palladia |
| AHFS/Drugs.com | Veterinary Use |
| License data | |
| Routes of administration | By mouth |
| Drug class | Antineoplastic agent |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 77% |
| Protein binding | 91%-93% |
| Elimination half-life | 16 h |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL |
|
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
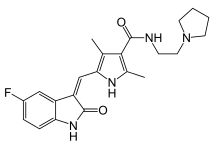
| Formula | C22H25FN4O2 |
| Molar mass | 396.466 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Toceranib (INN[2]), sold under the brand name Palladia, is a receptor tyrosine kinase inhibitor that is used in the treatment of canine mast cell tumor also called mastocytoma.[3] It is the first medication developed specifically for the treatment of cancer in dogs.[4][5] It is used as its phosphate salt, toceranib phosphate. It was developed by SUGEN as SU11654,[6] a sister compound to sunitinib, which was later approved for human therapies. Toceranib is a tyrosine kinase inhibitor and works in two ways: by killing tumor cells and by cutting off the blood supply to the tumor.[4]
The most common side effects include diarrhea, decrease or loss of appetite, lameness, weight loss, and blood in the stool.[4]
- ^ "Palladia EPAR". European Medicines Agency. 1 October 2009. Retrieved 1 July 2024.
- ^ World Health Organization (2009). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 62". WHO Drug Information. 23 (2). hdl:10665/74420.
- ^ London CA, Malpas PB, Wood-Follis SL, Boucher JF, Rusk AW, Rosenberg MP, et al. (June 2009). "Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision". Clinical Cancer Research. 15 (11): 3856–3865. doi:10.1158/1078-0432.CCR-08-1860. PMID 19470739.
- ^ a b c "FDA: First Drug to Treat Cancer in Dogs Approved". U.S. Food and Drug Administration (FDA) (Press release). 3 June 2009. Archived from the original on 22 July 2010. Retrieved 2 October 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Palladia New Animal Drug Application" (PDF). U.S. Food and Drug Administration (FDA). 22 May 2009. Archived from the original (PDF) on 16 November 2010. Retrieved 2 October 2021.
- ^ "In Trials for New Cancer Drugs, Family Pets Are Benefiting, Too". The New York Times. 24 November 2006. Archived from the original on 27 February 2021. Retrieved 2 October 2021.
