
Back قانون بويل Arabic Llei de Boyle-Mariotte AST Boyl-Mariott qanunu Azerbaijani Закон Бойля-Марыёта Byelorussian Закон на Бойл – Мариот Bulgarian বয়েলের সূত্র Bengali/Bangla Boyle-Mariotteov zakon BS Llei de Boyle Catalan یاسای بۆیڵ CKB Boyleův–Mariottův zákon Czech
The English used in this article or section may not be easy for everybody to understand. (May 2023) |
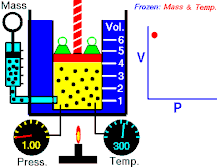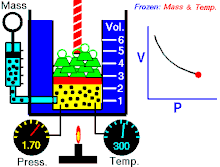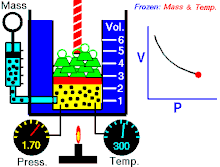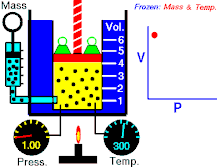
Boyle's law (also called Mariotte's law and the Boyle-Mariotte law) is a law about ideal gases.
The law can be stated as follows:[1]
For a fixed amount of an ideal gas kept at a fixed temperature, P (pressure) and V (volume) are inversely proportional.
In other words, the volume of a constant mass of ideal gas at a constant temperature is inversely proportional to the pressure applied on it.[2]
In symbols, the law is:
or
where P is the pressure of the gas, V is the volume of the gas, and k is a constant.
For a given mass of gas at a constant temperature, the product of the pressure and the volume is constant. As the volume decreases, the pressure increases in proportion, and vice versa. For example, when the pressure halves, the volume doubles.
Suppose you have a tank that contains a certain volume of gas at a certain pressure. When you decrease the volume of the tank, the same number of gas particles is now contained in a smaller space. Therefore, the number of collisions increases. Therefore, the pressure is greater.[3]
Imagine you have a gas at a certain pressure (P1) and volume (V1). If you change the pressure to a new value (P2), the volume changes to a new value (V2). We can use Boyle's law to describe both sets of conditions:[3]
The constant, k, is the same in both cases, so we can say the following:[3]
Example: The pressure of a gas is 3 atm and the volume is 5 litres. If the pressure is reduced to 2 atm, what is the volume?
∴ The volume will be 7.5 litres.
The law was found by Robert Boyle in 1662, and afterwards independently by Edme Mariotte in 1679.[2][4][5]
- ↑ Levine, Ira N. (2009). Physical Chemistry (Sixth ed.). New York: McGraw-Hill. p. 10. ISBN 978-0-07-253862-5. Archived from the original on 2020-11-30. Retrieved 2013-11-24.
- ↑ 2.0 2.1 Daintith, John, ed. (2008). A Dictionary of Chemistry (Sixth ed.). Oxford: Oxford University Press. p. 82. ISBN 978-0-19-920463-2.
- ↑ 3.0 3.1 3.2 Moore, John T. (2010). Chemistry Essentials For Dummies. New Jersey: John Wiley & Sons, Inc. p. 163. ISBN 978-0-470-61836-3.
- ↑ Ganot, Adolphe; Atkinson, Edmund (1883). Éléments de Physique [Elementary Treatise on Physics] (Eleventh ed.). London: Longmans, Green, and Co. p. 142.
- ↑ West, John B. (2005-01-01), "Robert Boyle's landmark book of 1660 with the first experiments on rarified air", Journal of Applied Physiology, 98 (1): 31–39, doi:10.1152/japplphysiol.00759.2004, PMID 15591301, S2CID 5837786[permanent dead link]








