
Back Waterstofbinding Afrikaans Vinclo d'hidrocheno AN رابطة هيدروجينية Arabic Fuercia per ponte d'hidróxenu AST Hidrogen rabitəsi Azerbaijani Вадародная сувязь Byelorussian Водородна връзка Bulgarian হাইড্রোজেন বন্ধন Bengali/Bangla Vodikova veza BS Enllaç per pont d'hidrogen Catalan
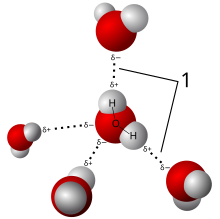
A hydrogen bond is a type of chemical bond that weakly attaches a molecule to another molecule. It is based on the attraction between opposite electric charges. The negative charge on an electronegative atom of one molecule is attracted to a positive charge on a hydrogen atom of another molecule. The hydrogen atom carries a positive charge because it is bonded to a second electronegative atom, which shifts electrons away from the hydrogen. This type of bond always involves a hydrogen atom, and two electronegative atoms. The electronegative atoms are often oxygen or nitrogen. Hydrogen bonds are important in polar solvents such as water and alcohol,[1] in biomolecules, and in many other materials.
Hydrogen bonds can occur between molecules (intermolecular bonds), or between different parts of a single molecule (intramolecular bonds).[2] The typical hydrogen bond is stronger than van der Waals forces, but weaker than covalent, ionic and metallic bonds.
- ↑ Breslyn, Wayne. "Hydrogen Bonding in Ethanol (C2H5OH)". Retrieved 31 May 2021.
- ↑ Compendium of Chemical Terminology, hydrogen bond, accessed 15 Jan 2007.