
Back تيروسين Arabic تيروسين ARZ تیروزین AZB Тирозин Bulgarian Tirozin BS Tirosina Catalan Tyrosin Czech Tyrosin Danish Tyrosin German Τυροσίνη Greek
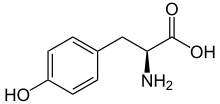 L-Tyrosine
| ||
 L-Tyrosine at physiological pH
| ||
| Names | ||
|---|---|---|
| IUPAC name
(S)-Tyrosine
| ||
| Other names
L-2-Amino-3-(4-hydroxyphenyl)propanoic acid
| ||
| Identifiers | ||
| ||
3D model (JSmol)
|
||
| ChEBI | ||
| ChEMBL | ||
| ChemSpider | ||
| DrugBank | ||
| ECHA InfoCard | 100.000.419 | |
PubChem CID
|
||
| UNII | ||
CompTox Dashboard (EPA)
|
||
| ||
| Properties | ||
| C9H11NO3 | ||
| Molar mass | 181.19 g·mol−1 | |
| .0453 g/100 mL | ||
| -105.3·10−6 cm3/mol | ||
| Hazards | ||
| NFPA 704 |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | ||
| Infobox references | ||
Tyrosine (Tyr or Y[1]) or 4-hydroxyphenylalanine is an amino acid.
Tyrosine is one of the 20 standard amino acids used by cells to make proteins. It is a non-essential amino acid, meaning the body can make it. Its codons are UAC and UAU.
Tyrosine can be synthesized in the body from phenylalanine. It is also found in many high-protein food products such as chicken, turkey, fish, milk, yogurt, cottage cheese, cheese, peanuts, almonds, pumpkin seeds, sesame seeds, soy products, lima beans, avocados, bananas and eggs.[2]
It is called tyrosyl when referred to as a functional group or side chain.[3]
- ↑ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
- ↑ "Tyrosine". University of Maryland Medical Center. Archived from the original on 2020-04-06. Retrieved 2011-03-17.
- ↑ "Amino Acids - Tyrosine". www.biology.arizona.edu. Retrieved 2018-01-31.
